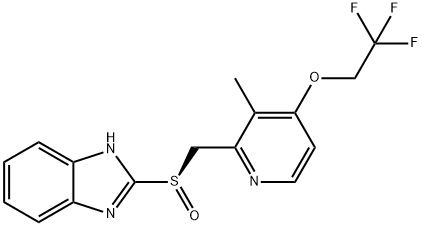
R-(+)-Lansoprazole
- CAS No.
- 138530-94-6
- Chemical Name:
- R-(+)-Lansoprazole
- Synonyms
- Dexlansoprazole;TAK 390;T 168390;D-lansoprazole;Dexlansaprazole;Dexlanzoprazole;T 168390,TAK 390;(+)-Lansoprazole;R-(+)-Lansoprazole;Lansoprazole R-Isomer
- CBNumber:
- CB71269821
- Molecular Formula:
- C16H14F3N3O2S
- Molecular Weight:
- 369.36
- MOL File:
- 138530-94-6.mol
- Modify Date:
- 2024/3/22 14:56:24
| Melting point | 66-68?C |
|---|---|
| Boiling point | 555.8±60.0 °C(Predicted) |
| Density | 1.50±0.1 g/cm3(Predicted) |
| storage temp. | Sealed in dry,2-8°C |
| solubility | Chloroform (Slightly), DMSO (Slightly), Methanol (Slightly) |
| form | Solid |
| pka | 9.56±0.10(Predicted) |
| color | Off-White to Dark Brown |
| Stability | Hygroscopic |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS08,GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H317-H373 | |||||||||
| Precautionary statements | P260-P314-P501-P261-P272-P280-P302+P352-P333+P313-P321-P363-P501 | |||||||||
| NFPA 704 |
|
R-(+)-Lansoprazole Chemical Properties,Uses,Production
Description
The mechanism of PPIs involves the irreversible binding to the hydrogen/potassium adenosine triphosphatase enzyme system, commonly referred to as the gastric proton pump, of the gastric parietal cell. As the last stage in gastric acid secretion, blockade of the gastric proton pump is an effective treatment for a variety of diseases requiring acid suppression, such as heartburn, peptic ulcers, and GERD. Dexlansoprazole is the latest PPI to hit the market, joining the ranks of omeprazole, rabeprazole, pantoprazole, esomeprazole, and lansoprazole, and is the Renantiomer of the racemic lansoprazole. Compared to its predecessors, dexlansoprazole exhibits improved pharmacokinetics with slower clearance and longer terminal half-life. In addition, dexlansoprazole utilizes a novel DDR technology; drug release is optimized through the use of granules with different pH-dependent dissolution profiles, thereby providing an initial release in the proximal small intestine within 1-2 h of administration followed by a subsequent release at distal regions of the small intestine several hours later. With its longer duration of action culminating in more effective acid suppression, dexlansoprazole may have an advantage over conventional PPIs that possess single release formulations (immediate or delayed). Similar to all PPIs, dexlansoprazole is a prodrug that consists of pyridine and benzimidazole rings with a latent sulfenamide moiety. In order to form the disulfide bond with cysteine residues of the proton pump, dexlansoprazole must be activated through two protonations followed by a spontaneous rearrangement to unmask the sulfenamide.
Chemical Properties
Brown Solid
Uses
The R-enantiomer of Lansoprazole; a gastric proton pump inhibitor. An antiulcerative
Clinical Use
Takeda Pharmaceuticals received approval of dexlansoprazole, a dual release formulation of the (R)-isomer of lansoprazol proton pump inhibitor (PPI) already in the market, from the FDA in January 2009. Dexlansoprazole is a delayed release capsule for the oncedaily, oral treatment of heartburn associated with symptomatic non-erosive gastroesophageal reflux disease (GERD), the healing of erosive esophagitis (EE) and the maintenance of healed EE. The dual release formulation is designed to provide two separate releases of medication, one at 1–2 h and then another at 4–5 h after treatment, for extended efficacy in the treatment of GERD.
Side effects
The most commonly recorded adverse reactions that occurred at a higher incidence than placebo were diarrhea, abdominal pain, nausea, vomiting, flatulence, and upper respiratory tract infection. As dexlansoprazole inhibits gastric acid secretion, its use is expected to interfere with the absorption of drugs with pH-dependent oral bioavailability. Since the HIV protease inhibitor atazanavir is dependent on gastric acid for absorption, dexlansoprazole should not be co-administered with atazanavir to avoid a loss of therapeutic efficacy. While co-administration of dexlansoprazole did not affect the pharmacokinetics of warfarin or INR (international normalized ratio: the ratio of a patient s prothrombin time to a normal sample), there have been reports of increased INR and prothrombin time in patients receiving concomitant treatment with PPIs and warfarin. Since increases in INR and prothrombin time may lead to abnormal bleeding and possibly death, concomitant use of dexlansoprazole and warfarin may necessitate monitoring for increases in INR and prothrombin time.
R-(+)-Lansoprazole Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| TAGOOR LABORATORIES PVT LTD | +919700187008 | AndhraPradesh, India | 93 | 58 | Inquiry |
| RP Industries | +91-9700187008 +91-9700187008 | Gujarat, India | 58 | 58 | Inquiry |
| GLP Pharma Standards | +91 9866074638 | Hyderabad, India | 1644 | 58 | Inquiry |
| Metrochem API Private Limited | +91-4069069999 +91-4069069999 | Telangana, India | 78 | 58 | Inquiry |
| Sekhment pharmaventures | +91-9030088669 +91-9030088669 | Mumbai, India | 105 | 58 | Inquiry |
| Apotex Pharmachem India Pvt Ltd | +91-8022891034 +91-8022891000 | Karnataka, India | 109 | 58 | Inquiry |
| Piramal Pharma Solutions | +919892735994 | Maharashtra, India | 48 | 58 | Inquiry |
| Lupin Ltd | +91-8019896181 +91-8019896181 | Maharashtra, India | 93 | 58 | Inquiry |
| Chemeca Drugs Private Limited (Vegesna Laboratories Pvt Ltd) | +91-8924236065 +91-9951616502 | AndhraPradesh, India | 48 | 58 | Inquiry |
| Enal Drugs Pvt Ltd | +914044753636 | Telangana, India | 35 | 58 | Inquiry |
138530-94-6(R-(+)-Lansoprazole)Related Search:
1of4
chevron_right




