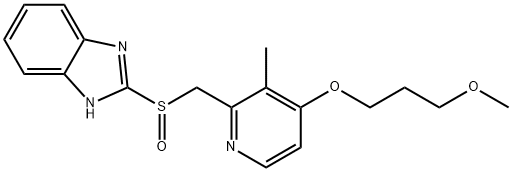
Rabeprazole
- CAS No.
- 117976-89-3
- Chemical Name:
- Rabeprazole
- Synonyms
- Rebeprazole;CS-1363;NSC24156;ly-307640;NSC 24156;NSC-24156;RABEPRAZOLE;Rabeprazole USP/EP/BP;Rabeprazole-IP/USP/IHS;Rabeprazole, LY 307640
- CBNumber:
- CB3680731
- Molecular Formula:
- C18H21N3O3S
- Molecular Weight:
- 359.44
- MOL File:
- 117976-89-3.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/6/27 13:31:27
| Melting point | 99-100° (dec) |
|---|---|
| Boiling point | 603.9±65.0 °C(Predicted) |
| Density | 1.33±0.1 g/cm3(Predicted) |
| storage temp. | Inert atmosphere,Store in freezer, under -20°C |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| form | Solid |
| pka | 9.57±0.10(Predicted) |
| color | Light Brown to Very Dark Brown |
| BCS Class | 1,3 |
| Stability | Hygroscopic |
| CAS DataBase Reference | 117976-89-3(CAS DataBase Reference) |
Rabeprazole Chemical Properties,Uses,Production
Description
Rabeprazole, a proton pump inhibitor (PPI), effectively lowers stomach acid production to alleviate conditions such as gastroesophageal reflux disease (GERD), ulcers, and other issues caused by excessive acid production. It belongs to the benzimidazole derivative family, sharing a structural similarity with omeprazole, another PPI. Rabeprazole hampers the activity of the gastric proton pump responsible for acid production. By binding to the pump, it obstructs the release of hydrogen ions into the stomach, ultimately reducing acid production. Furthermore, rabeprazole exhibits various biochemical and physiological effects. A study conducted on rats revealed that rabeprazole effectively decreases the gastric proton pump′s activity, leading to a decrease in gastric acid secretion.
brand name
Aciphex (Eisai Medical Research) .
Mechanism of action
Rabeprazole is a proton pump inhibitor (PPI) and as such covalently binds with and inactivates the gastric parietal cell proton pump (H+/K+-ATPase). This inhibits in turn gastric acid production and raises gastric pH[1].
Pharmacokinetics
Rabeprazole is marketed as an enterically coated formulation due to the instability of all PPIs in an acid environment. After oral ingestion, it is relatively rapidly absorbed as the maximal plasma concentration reaches between 2.8 and 5.1 postdose. The pharmacokinetics of the molecule is linear in the range 10–80 mg with an overall bioavailability of 52%. Rabeprazole does not have a saturable first-pass metabolism, and it can be absorbed in high doses.
Neither antacids nor food influences the bioavailability of the molecule, even if food intake delayed the absorption of rabeprazole 20 mg of about 1.7 h and reduced the apparent elimination half-life due to a probable delayed gastric emptying. Preclinical studies have demonstrated that rabeprazole’s volume of distribution is 0.34 L/kg in various tissues, including the gastric mucosa, stomach, kidney, bladder, liver, intestine, and thyroid. In healthy volunteers, it was demonstrated to be bound to 94.8%–97.5% of plasma proteins.
Side effects
The more common side effects of rabeprazole can include: headache. pain in the abdomen (stomach area) sore throat.
The serious side effects of rabeprazole can include: has stomach cramps, bloated feeling, watery and severe diarrhea which may also be bloody sometimes, fever, nausea or vomiting, or unusual tiredness or weakness. This medicine may increase your risk of having fractures of the hip, wrist, and spine.
Drug interactions
A product that may interact with this drug is: methotrexate (especially high-dose treatment).
Some products need stomach acid so that the body can absorb them properly. Rabeprazole decreases stomach acid, so it may change how well these products work. Some affected products include ampicillin, atazanavir, erlotinib, levoketoconazole, nelfinavir, pazopanib, rilpivirine, sparsentan, certain azole antifungals (itraconazole, ketoconazole, posaconazole), among others.
References
[1] Fabio Pace. “A review of rabeprazole in the treatment of acid-related diseases.” Therapeutics and Clinical Risk Management 3 3 (2007): 363–79.
Rabeprazole Preparation Products And Raw materials
Raw materials
1of2
chevron_rightPreparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| SGMR PHARMACEUTICALS PVT LTD | +91-9032001889 +91-9032001889 | Telangana, India | 83 | 58 | Inquiry |
| ANWITA APIS | +919000311012 | Hyderabad, India | 198 | 58 | Inquiry |
| TYNDAL LABS PVT LTD | +91-8008166674 +91-8008166674 | AndhraPradesh, India | 95 | 58 | Inquiry |
| Synaptics Labs Private Limited | +91-8341963666 +91-8341963666 | Hyderabad, India | 84 | 58 | Inquiry |
| Ions Pharma | +912266707500 | Maharashtra, India | 89 | 58 | Inquiry |
| Sainor Life Sciences Pvt Ltd | +919542990009 | AndhraPradesh, India | 13 | 58 | Inquiry |
| Lakshmi Farmachem | +91-9948795885 +91-9550886476 | Telangana, India | 407 | 58 | Inquiry |
| Indo Rama Pharma | +91-9215466664 +91-9315564742 | Himachal Pradesh, India | 1 | 58 | Inquiry |
| Ralington Pharma | +91-7948911722 +91-9687771722 | Gujarat, India | 1350 | 58 | Inquiry |
| PROTECH TELELINKS | +91-8571891912 +91-9855060837 | Himachal Pradesh, India | 62 | 58 | Inquiry |
| Supplier | Advantage |
|---|---|
| SGMR PHARMACEUTICALS PVT LTD | 58 |
| ANWITA APIS | 58 |
| TYNDAL LABS PVT LTD | 58 |
| Synaptics Labs Private Limited | 58 |
| Ions Pharma | 58 |
| Sainor Life Sciences Pvt Ltd | 58 |
| Lakshmi Farmachem | 58 |
| Indo Rama Pharma | 58 |
| Ralington Pharma | 58 |
| PROTECH TELELINKS | 58 |
Related Qustion
- Q:Is Rabeprazole more effective compared to omeprazole and pantoprazole?
- A:Yes. Rabeprazole is a proton pump inhibitor (PPI) with potent and long-acting inhibition of gastric acid secretion.
- Jun 11,2024
117976-89-3(Rabeprazole)Related Search:
1of4
chevron_right





