Pantoprazole
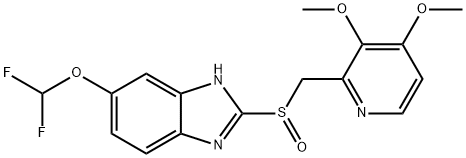
- CAS No.
- 102625-70-7
- Chemical Name:
- Pantoprazole
- Synonyms
- BY-1023;SKF-96022;Patoprazole;PANTOPRAZOLE;Pantoprozole;Pantoprazole D7;Pantoprazole&Int.;Pantoprazole Acid;Pantoprazole (BY1023);Pantoprazole USP/EP/BP
- CBNumber:
- CB8129842
- Molecular Formula:
- C16H15F2N3O4S
- Molecular Weight:
- 383.37
- MOL File:
- 102625-70-7.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/3/16 7:03:48
| Melting point | 139-140° (dec) |
|---|---|
| Boiling point | 586.9±60.0 °C(Predicted) |
| Density | 1.51±0.1 g/cm3(Predicted) |
| storage temp. | Inert atmosphere,Store in freezer, under -20°C |
| solubility | Chloroform (Slightly), Methanol (Slightly, Heated, Sonicated) |
| pka | pKa1 3.92; pKa2 8.19(at 25℃) |
| form | Solid |
| color | Off-White to Pale Orange |
| BCS Class | 3 |
| InChI | InChI=1S/C16H15F2N3O4S/c1-23-13-5-6-19-12(14(13)24-2)8-26(22)16-20-10-4-3-9(25-15(17)18)7-11(10)21-16/h3-7,15H,8H2,1-2H3,(H,20,21) |
| InChIKey | IQPSEEYGBUAQFF-UHFFFAOYSA-N |
| SMILES | C1(S(CC2=NC=CC(OC)=C2OC)=O)NC2=CC(OC(F)F)=CC=C2N=1 |
| CAS DataBase Reference | 102625-70-7(CAS DataBase Reference) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H302+H312+H332 | |||||||||
| Hazard Codes | Xn | |||||||||
| Risk Statements | 20/21/22-37/38-41-48 | |||||||||
| Safety Statements | 22-26-36/37/39-45 | |||||||||
| NFPA 704 |
|
Pantoprazole Chemical Properties,Uses,Production
Description
Pantoprazole, an irreversible proton pump inhibitor, reached its first market worldwide in Germany for acute treatment of gastric and duodenal ulcers and gastroesophageal reflux disease. Proton pump inhibitors are more effective than other strategies in inhibiting acid secretion since they function at the final step of acid production, therefore, provide superior symptom relief and healing in all acid related diseases. As the third substituted benzimidazole proton pump inhibitor marketed, pantoprazole not only has superior ulcer-preventing effect but also is more potent than omeprazole in healing acetic acid-induced gastric and duodenal ulcers with extremely low acute toxicity. The mechanism of action for this class of compounds has been suggested to be mediated via the protonated form of the molecule which selectively reacts with cysteines present on the extracytoplasmic face of the enzyme to form covalent disulfide bonds.
Uses
proton pump inhibitor, gastric acid release inhibitor, antiulcer
Definition
ChEBI: A member of the class of benzimidazoles that is 1H-benzimidazole substituted by a difluoromethoxy group at position 5 and a [(3,4-dimethoxypyridin-2-yl)methyl]sulfinyl group at position 2.
Pantoprazole Preparation Products And Raw materials
Raw materials
1of2
chevron_rightPreparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Jigs Chemical ltd | +919099003427 | Gujarat, India | 239 | 58 | Inquiry |
| ANWITA APIS | +919000311012 | Hyderabad, India | 198 | 58 | Inquiry |
| KARPSCHEM LABORATORIES | +91-7249203006 +91-7249203006 | Maharashtra, India | 786 | 58 | Inquiry |
| Metrochem API Private Limited | +91-4069069999 +91-4069069999 | Telangana, India | 78 | 58 | Inquiry |
| Dodhia Group | +91-9619867345 +91-9619867345 | Mumbai, India | 129 | 58 | Inquiry |
| Sainor Life Sciences Pvt Ltd | +919542990009 | AndhraPradesh, India | 13 | 58 | Inquiry |
| Prachi Pharmaceuticals Pvt Ltd | +919004877711 | Mumbai, India | 36 | 58 | Inquiry |
| Macleods Pharmaceuticals Limited | +91-2266762800 +91-2266762800 | Maharashtra, India | 116 | 58 | Inquiry |
| Lakshmi Farmachem | +91-9948795885 +91-9550886476 | Telangana, India | 407 | 58 | Inquiry |
| Ralington Pharma | +91-7948911722 +91-9687771722 | Gujarat, India | 1350 | 58 | Inquiry |
| Supplier | Advantage |
|---|---|
| Jigs Chemical ltd | 58 |
| ANWITA APIS | 58 |
| KARPSCHEM LABORATORIES | 58 |
| Metrochem API Private Limited | 58 |
| Dodhia Group | 58 |
| Sainor Life Sciences Pvt Ltd | 58 |
| Prachi Pharmaceuticals Pvt Ltd | 58 |
| Macleods Pharmaceuticals Limited | 58 |
| Lakshmi Farmachem | 58 |
| Ralington Pharma | 58 |





