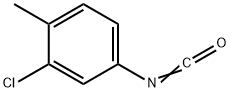
3-Chloro-4-methylphenyl isocyanate
- CAS No.
- 28479-22-3
- Chemical Name:
- 3-Chloro-4-methylphenyl isocyanate
- Synonyms
- 3-Chlor-p-tolylisocyanat;3-chloro-p-tolyl isocyanate;2-CHLORO-4-ISOCYANATO-TOLUENE;3-Chloro-4-MethylPhenylIsocynate;3-CHLORO-4-METHYLPHENYL ISOCYANATE;3-Chlore-4-methylphenyl isocyanate;Isocyanato-3-chloro-4-methyl benzene;3-Chloro-4-methylphenylIsocyanate>3-Chloro-4-methylphenyl isocyanate-98%;3-Chloro-4-methylphenyl isocyanate ,99%
- CBNumber:
- CB7172841
- Molecular Formula:
- C8H6ClNO
- Molecular Weight:
- 167.59
- MOL File:
- 28479-22-3.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/3/14 15:18:27
| Melting point | 20-24℃ |
|---|---|
| Boiling point | 107 °C/3 mmHg (lit.) |
| Density | 1.224 g/mL at 25 °C (lit.) |
| vapor pressure | 13.5Pa at 25℃ |
| refractive index |
n |
| Flash point | 229 °F |
| storage temp. | 2-8°C |
| form | Liquid or Low Melting Solid |
| color | Colorless to yellow |
| Sensitive | Moisture Sensitive |
| BRN | 2690771 |
| LogP | 3.78 |
| CAS DataBase Reference | 28479-22-3(CAS DataBase Reference) |
| EPA Substance Registry System | 3-Chloro-4-methylphenyl isocyanate (28479-22-3) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |     GHS05,GHS06,GHS08,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H302+H312-H314-H317-H330-H334-H335-H410 | |||||||||
| Precautionary statements | P273-P280-P301+P312-P303+P361+P353-P304+P340+P310-P305+P351+P338 | |||||||||
| Hazard Codes | Xn | |||||||||
| Risk Statements | 20/21/22-36/37/38-42 | |||||||||
| Safety Statements | 23-26-36/37-45 | |||||||||
| RIDADR | UN 2206 6.1/PG 3 | |||||||||
| WGK Germany | 1 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 6.1 | |||||||||
| PackingGroup | II | |||||||||
| HS Code | 29291090 | |||||||||
| NFPA 704 |
|
3-Chloro-4-methylphenyl isocyanate price More Price(6)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | 478245 | 3-Chloro-4-methylphenyl isocyanate 98% | 28479-22-3 | 5G | ₹3897 | 2022-06-14 | Buy |
| TCI Chemicals (India) | I0563 | 3-Chloro-4-methylphenyl Isocyanate min. 97.0 % | 28479-22-3 | 5G | ₹2900 | 2022-05-26 | Buy |
| ALFA India | ALF-L12064-14 | 3-Chloro-4-methylphenyl isocyanate, 98% | 28479-22-3 | 25g | ₹12800 | 2022-05-26 | Buy |
| TCI Chemicals (India) | I0563 | 3-Chloro-4-methylphenyl Isocyanate min. 97.0 % | 28479-22-3 | 25G | ₹12800 | 2022-05-26 | Buy |
| ALFA India | ALF-L12064-06 | 3-Chloro-4-methylphenyl isocyanate, 98% | 28479-22-3 | 5g | ₹2660 | 2022-05-26 | Buy |
3-Chloro-4-methylphenyl isocyanate Chemical Properties,Uses,Production
Chemical Properties
Chloromethyl phenyl isocyanate is a colorless to yellow liquid or beige, low-melting solid. Acrid odor.
Uses
3-Chloro-4-methylphenyl isocyanate may be used in the synthesis of the following urea compounds:
- 1-(1-(1-adamantyl)methyl)-3-(3-chloro-4-methylphenyl)urea
- 1-(3-chloro-4-methylphenyl)-3-heptylurea
- 1-(3-chloro-4-methylphenyl)-3-cyclooctylurea
- 1-(3-chloro-4-methylphenyl)-3-(3-fluorobenzyl)urea
- 1-(3-chloro-4-methylphenyl)-3-(4-phenylbutan-2-yl)urea
General Description
A colorless liquid with an acrid odor. Denser than water. Contact may irritate skin, eyes and mucous membranes. Flash point 132°F. Toxic by ingestion, inhalation and skin absorption. Used to make other chemicals.
Air & Water Reactions
Flammable. 3-Chloro-4-methylphenyl isocyanate decomposes in water. Substance will react with water (some violently) releasing flammable, toxic or corrosive gases and runoff.
Reactivity Profile
Isocyanates and thioisocyanates, such as 3-Chloro-4-methylphenyl isocyanate, are incompatible with many classes of compounds, reacting exothermically to release toxic gases. Reactions with amines, aldehydes, alcohols, alkali metals, ketones, mercaptans, strong oxidizers, hydrides, phenols, and peroxides can cause vigorous releases of heat. Acids and bases initiate polymerization reactions in these materials. Some isocyanates react with water to form amines and liberate carbon dioxide. Base-catalysed reactions of isocyanates with alcohols should be carried out in inert solvents. Such reactions in the absence of solvents often occur with explosive violence, [Wischmeyer(1969)].
Health Hazard
TOXIC; inhalation, ingestion or contact (skin, eyes) with vapors, dusts or substance may cause severe injury, burns or death. Contact with molten substance may cause severe burns to skin and eyes. Reaction with water or moist air will release toxic, corrosive or flammable gases. Reaction with water may generate much heat that will increase the concentration of fumes in the air. Fire will produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
Fire Hazard
Combustible material: may burn but does not ignite readily. Substance will react with water (some violently) releasing flammable, toxic or corrosive gases and runoff. When heated, vapors may form explosive mixtures with air: indoors, outdoors and sewers explosion hazards. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapors may travel to source of ignition and flash back. Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated or if contaminated with water.
Potential Exposure
This material is used in organic synthesis.
Shipping
UN22363-Chloro-4-methylphenyl isocyanate, liquid, Hazard Class: 6.1; Labels: 6.1-Poisonous materials.
Incompatibilities
May form explosive mixture with air. Isocyanates are highly flammable and reactive with many compounds, even with themselves. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Reaction with moist air, water or alcohols may form amines and insoluble polyureas and react exothermically, releasing toxic, corrosive, or flammable gases, including carbon dioxide; and, at the same time, may generate a violent release of heat increasing the concentration of fumes in the air. Incompatible with amines, aldehydes, alkali metals, ammonia, carboxylic acids, caprolactum, alkaline materials, glycols, ketones, mercaptans, hydrides, organotin catalysts, phenols, strong acids, strong bases, strong reducing agents such as hydrides, urethanes, ureas. Elevated temperatures or contact with acids, bases, tertiary amines, and acyl-chlorides may cause explosive polymerization. Attacks some plastics, rubber, and coatings. Contact with metals may evolve flammable hydrogen gas. May accumulate static electrical charges, and may cause ignition of its vapors. Do not store in temperatures above 30C/86F.
3-Chloro-4-methylphenyl isocyanate Preparation Products And Raw materials
Raw materials
1of2
chevron_rightPreparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| LANXESS India Pvt. Ltd. | +91-8356878763 +91-8356878763 | Maharashtra, India | 190 | 58 | Inquiry |
| SREE SYNERIC LAB | null | New Delhi, India | 328 | 58 | Inquiry |
| Siddhi Specialities | 91-0-9742033800 | Bangalore, India | 162 | 58 | Inquiry |
| TCI Chemicals (India) Pvt. Ltd. | 1800 425 7889 | New Delhi, India | 6778 | 58 | Inquiry |
| A.J Chemicals | 91-9810153283 | New Delhi, India | 6124 | 58 | Inquiry |
| Alfa Aesar | 1 800 209 7001 | Maharashtra, India | 6913 | 58 | Inquiry |
| LANXESS India Pvt. Ltd. | +91-22-2587 1000 | Maharashtra, India | 131 | 58 | Inquiry |
| career henan chemical co | +86-0371-86658258 +8613203830695 | China | 29900 | 58 | Inquiry |
| Chongqing Chemdad Co., Ltd | +86-023-6139-8061 +86-86-13650506873 | China | 39916 | 58 | Inquiry |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | China | 49391 | 58 | Inquiry |
| Supplier | Advantage |
|---|---|
| LANXESS India Pvt. Ltd. | 58 |
| SREE SYNERIC LAB | 58 |
| Siddhi Specialities | 58 |
| TCI Chemicals (India) Pvt. Ltd. | 58 |
| A.J Chemicals | 58 |
| Alfa Aesar | 58 |
| LANXESS India Pvt. Ltd. | 58 |
| career henan chemical co | 58 |
| Chongqing Chemdad Co., Ltd | 58 |
| CONIER CHEM AND PHARMA LIMITED | 58 |
28479-22-3(3-Chloro-4-methylphenyl isocyanate)Related Search:
1of4
chevron_right




