LITHIUM METABORATE
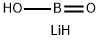
- CAS No.
- 13453-69-5
- Chemical Name:
- LITHIUM METABORATE
- Synonyms
- Boricacidlithiumsalt;SPECTROMELT C 20;SPECTROMELT A 20;Lithiummetaborat;ithium metaborate;LITHIUM METABORATE;SPECTROMELT(R) C20;LITHIUM BORATE (META);LITHIUM METABORATE AR;lithium metaborate, acs
- CBNumber:
- CB7208760
- Molecular Formula:
- BH2LiO2
- Molecular Weight:
- 51.76
- MOL File:
- 13453-69-5.mol
- MSDS File:
- SDS
- Modify Date:
- 2025/1/27 9:38:02
| Melting point | 845 °C (lit.) |
|---|---|
| bulk density | 1300kg/m3 |
| Density | 1,4 g/cm3 |
| storage temp. | Store at +5°C to +30°C. |
| solubility | soluble in ethanol. |
| form | Powder |
| Specific Gravity | 1.4 |
| color | White |
| Water Solubility | Soluble in water(27.5g/L). |
| Sensitive | Hygroscopic |
| Exposure limits | ACGIH: TWA 2 mg/m3; STEL 6 mg/m3 |
| Stability | Stable. |
| CAS DataBase Reference | 13453-69-5(CAS DataBase Reference) |
| EPA Substance Registry System | Lithium metaborate (13453-69-5) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS05,GHS07,GHS08 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H302-H318-H361fd | |||||||||
| Precautionary statements | P202-P264-P280-P301+P312-P305+P351+P338-P308+P313 | |||||||||
| Risk Statements | 20/21/22-41 | |||||||||
| Safety Statements | 24/25 | |||||||||
| WGK Germany | 1 | |||||||||
| F | 3 | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 2840 20 90 | |||||||||
| NFPA 704 |
|
LITHIUM METABORATE price More Price(15)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | 402966 | Lithium metaborate ACS reagent, ≥98.0% | 13453-69-5 | 100G | ₹10164.68 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 205524 | Lithium metaborate 99.9% trace metals basis | 13453-69-5 | 100G | ₹3864.53 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 254282 | Lithium metaborate 99.995% trace metals basis | 13453-69-5 | 10G | ₹5087.75 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 205524 | Lithium metaborate 99.9% trace metals basis | 13453-69-5 | 500G | ₹16421.53 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 1.12996 | Spectromelt® A 20 lithium metaborate | 13453-69-5 | 1KG | ₹82209.99 | 2022-06-14 | Buy |
LITHIUM METABORATE Chemical Properties,Uses,Production
Chemical Properties
White powder
Uses
Lithium metaborate is used for flux.
Preparation
Lithium metaborate, LiBO2, may be prepared by fusing together either lithium hydroxide or lithium carbonate and boric acid in the proper stoichiometric ratio. Recrystallization from water at about room temperature yields lithium metaborate- octahydrate, LiBO2·8H2O. The octahydrate may be dried to yield a dihydrate, LiBO2-2H2O, or a half-hydrate, LiBO2·0.5H2O.
General Description
Lithium metaborate (LiBO2) is the lithium salt of boric acid. It can be synthesized by reacting orthoboric acid with lithium carbonate. Its crystals belong to the monoclinic crystal system having space group P21/c. Three polymorphic forms have been identified on ambient pressure devitrification of LiBO2: α-, γ- and β′-LiBO2.
Structure and conformation
Lithium metaborate has several crystal forms.
Below 100 °C, LiBO2·8H2O is firstly dehydrated to a lower hydrate LiBO2·2H2O [1].
When the temperature is higher than 190°C, the dehydrated amorphous hydrate could crystallize into other poorly crystalline phases designated as LiBO2·xH2O (x=0.3/0.5)[2].
An anhydrous monoclinic α-LiBO2 could be obtained when the baking temperature reaches 600 °C. The α form consists of infinite chains of trigonal planar metaborate anions [BO2O-]n.
At 950 °C, by applying 15 kbar of pressure to a LiCl flux containing α-LiBO2, α-LiBO2 could be converted to metastable γ-LiBO2 crystals which were small, but of high quality[3]. γ-LiBO2 has tetragonal symmetry with lattice parameter a=4.1961 ?, c=6.5112 ?, space group I-42d and a density of 2.882 g/cm3.
References
[1] E.I.Kamitsos, A.P.Patsis, M.A.Karakassides, G.D.Chryssikos, Infrared reflectance spectra of lithium borate glasses, Journal of Non-Crystalline Solids, 1990, 126, 52-67.
[2] Li Lei, Duanwei He, Kai He, Jiaqian Qin, Shanmin Wang, Pressure-induced coordination changes in LiBO2, Journal of Solid State Chemistry, 2009, 182, 3041-3048.
[3] Colin D. McMillen, Henry G. Giesber, Joseph W. Kolis, The hydrothermal synthesis, growth, and optical properties of γ-LiBO2, Journal of Crystal Growth, 310, 2008, 299-305.
LITHIUM METABORATE Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| GLR Innovations | +91 9891111994 | New Delhi, India | 4535 | 58 | Inquiry |
| Parad Corporation Pvt Ltd | +91-7433900100 +91-9426510550 | Gujarat, India | 37 | 58 | Inquiry |
| ARRAKIS INDUSTRIES LLP | +91 74995 32711 | Maharashtra, India | 1353 | 58 | Inquiry |
| A. B. Enterprises | 08048986961 | Mumbai, India | 226 | 58 | Inquiry |
| Otto Chemie Pvt. Ltd. | +91 9820041841 | Mumbai, India | 5870 | 58 | Inquiry |
| Alfa Aesar | 1 800 209 7001 | Maharashtra, India | 6905 | 58 | Inquiry |
| Central Drug House(P) Ltd. | 91-11-49404040 | New Delhi, India | 6157 | 58 | Inquiry |
| Triveni chemicals | 08048762458 | New Delhi, India | 6088 | 58 | Inquiry |
| LOBA CHEMIE PVT.LTD. | 91-22-6663 6699 | Mumbai, India | 3075 | 58 | Inquiry |
| C. H. Chemicals | 08048361348Ext 898 | Mumbai, India | 1 | 58 | Inquiry |
| Supplier | Advantage |
|---|---|
| GLR Innovations | 58 |
| Parad Corporation Pvt Ltd | 58 |
| ARRAKIS INDUSTRIES LLP | 58 |
| A. B. Enterprises | 58 |
| Otto Chemie Pvt. Ltd. | 58 |
| Alfa Aesar | 58 |
| Central Drug House(P) Ltd. | 58 |
| Triveni chemicals | 58 |
| LOBA CHEMIE PVT.LTD. | 58 |
| C. H. Chemicals | 58 |
Related articles
- Lithium Metaborate: Applications in Analytical Techniques and Safety Hazards
- Lithium metaborate's diverse applications and potential in technology are promising, yet safety precautions are crucial due to....
- Dec 2,2024
- What is lithium metaborate?
- Since lithium borate systems contain many important nonlinear optical crystals, it is important to study their structure and u....
- Aug 28,2023





