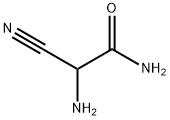
2-Amino-2-cyanoacetamide
- CAS No.
- 6719-21-7
- Chemical Name:
- 2-Amino-2-cyanoacetamide
- Synonyms
- ACA;2-aminocyanoacetamide;Aminocyanoacetamide;TIMTEC-BB SBB001501;3-Nitriloalaninamide;2-Amino-2-cyanacetamid;2-cyano-2-aminoacetamide;2-AMINO-2-CYANOACETAMIDE;ACETAMIDE, 2-AMINO-2-CYANO-;Amino(carbamoyl)acetonitrile
- CBNumber:
- CB7327490
- Molecular Formula:
- C3H5N3O
- Molecular Weight:
- 99.09
- MOL File:
- 6719-21-7.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/5/22 11:23:44
| Melting point | 120-124 °C |
|---|---|
| Boiling point | 338.5±37.0 °C(Predicted) |
| Density | 1.298±0.06 g/cm3(Predicted) |
| storage temp. | Store at <= 20°C. |
| pka | 17.05±0.50(Predicted) |
| form | Powder |
| color | Beige to tan |
| Water Solubility | soluble |
| InChI | InChI=1S/C3H5N3O/c4-1-2(5)3(6)7/h2H,5H2,(H2,6,7) |
| InChIKey | JRWAUKYINYWSTA-UHFFFAOYSA-N |
| SMILES | C(N)(=O)C(N)C#N |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS07,GHS09 |
|---|---|
| Signal word | Warning |
| Hazard statements | H312+H332-H315-H319-H335-H411 |
| Precautionary statements | P261-P273-P280-P302+P352+P312-P304+P340+P312-P305+P351+P338 |
| Hazard Codes | T,Xi,Xn |
| Risk Statements | 34-23-51-36-22 |
| Safety Statements | 45-36/37/39-26 |
| WGK Germany | WGK 1 slightly water endangering |
| Hazard Note | Irritant |
| HS Code | 2926 90 70 |
| Toxicity | LD50 orally in Rabbit: > 2000 mg/kg |
2-Amino-2-cyanoacetamide price More Price(2)
2-Amino-2-cyanoacetamide Chemical Properties,Uses,Production
Description
2-Amino-2-cyanoacetamide, a purine derivative with a diatomic molecule, has been shown to inhibit cancer cells in animals. It has been shown to inhibit the replication of plague virus, an RNA virus that causes encephalitis and other diseases in humans. The nitrogen atom on the molecule is responsible for its transport properties, including intestinal uptake and the ability to cross the blood-brain barrier.2-Amino-2-cyanoacetamide also inhibits the activity of amide hydrolase, an enzyme responsible for the hydrolysis of peptides into amino acids. This process results in reduced protein synthesis, which leads to cell death.
Chemical Properties
Beige Powder
Uses
2-Amino-2-cyanoacetamide is used as building block in chemical synthesis. Attributes Development Product Contact
Solubility in organics
soluble in ether and methanol.
2-Amino-2-cyanoacetamide Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Stereokem Pvt Ltd | +91-4029706929 +91-9394224843 | Telangana, India | 350 | 58 | Inquiry |
| Stereokem, Inc. | 500039 | New Delhi, India | 293 | 58 | Inquiry |
| A.J Chemicals | 91-9810153283 | New Delhi, India | 6124 | 58 | Inquiry |
| Alfa Aesar | 1 800 209 7001 | Maharashtra, India | 6913 | 58 | Inquiry |
| Srinivasa Labs | 09515114455 | Hyderabad, India | 113 | 58 | Inquiry |
| Metropolitan Eximchem Pvt., Ltd. (Metropolitan Group) | 91-22-24081528 | Maharashtra, India | 70 | 58 | Inquiry |
| JUYE XIANDAI fine chemistry Co.,Ltd | +86-18958038633; +8618958038633 | China | 64 | 58 | Inquiry |
| Shanghai Daken Advanced Materials Co.,Ltd | +86-371-66670886 | China | 16209 | 58 | Inquiry |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | China | 21675 | 55 | Inquiry |
| Hangzhou FandaChem Co.,Ltd. | 008657128800458; +8615858145714 | China | 9338 | 55 | Inquiry |
6719-21-7(2-Amino-2-cyanoacetamide)Related Search:
1of4
chevron_right




