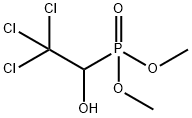
Trichlorfon
- CAS No.
- 52-68-6
- Chemical Name:
- Trichlorfon
- Synonyms
- DEP;TRICHLORPHON;METRIFONATE;Trichlorophon;dimethyl ester;Dipterex;TRICHLOROFON;chlorophos;DETF;Neguvon
- CBNumber:
- CB7472545
- Molecular Formula:
- C4H8Cl3O4P
- Molecular Weight:
- 257.44
- MOL File:
- 52-68-6.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/3/19 15:37:50
| Melting point | 77-81 °C |
|---|---|
| Boiling point | 100°C |
| Density | 1.73 |
| vapor pressure | 2.1×10-4Pa (20 °C) |
| refractive index | 1.3439 |
| storage temp. | 2-8°C |
| solubility | Freely soluble in water, very soluble in methylene chloride, freely soluble in acetone and in ethanol (96 per cent). |
| form | solid |
| pka | 6 (est.) |
| color | Crystals |
| Water Solubility | Slightly soluble. 1-5 g/100 mL at 21 ºC |
| Merck | 13,9696 |
| BRN | 1709434 |
| Stability | Light Sensitive |
| IARC | 3 (Vol. 30, Sup 7) 1987 |
| NIST Chemistry Reference | Metrifonate(52-68-6) |
| EPA Substance Registry System | Trichlorfon (52-68-6) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS07,GHS08,GHS09 |
|---|---|
| Signal word | Danger |
| Hazard statements | H302+H312-H317-H334-H410 |
| Precautionary statements | P273-P280-P301+P312+P330-P302+P352+P312 |
| Hazard Codes | Xn,N |
| Risk Statements | 22-43-50/53 |
| Safety Statements | 24-37-60-61-2 |
| RIDADR | UN 3077 9/PG 3 |
| WGK Germany | 3 |
| RTECS | TA0700000 |
| HazardClass | 6.1(b) |
| PackingGroup | III |
| HS Code | 29319090 |
| Toxicity | LD50 in male, female rats (mg/kg): 630, 560 orally (Gaines) |
Trichlorfon Chemical Properties,Uses,Production
Description
Trichlorfon is a colorless crystalline powder. It is soluble in water (120 g/L) and most organic solvents, except aliphatic hydrocarbons. Log Kow = 0.43. Trichlorfon is rapidly converted to dichlorvos by alkalis (2) and then hydrolyzed; DT50 (22 ?C) values at pH 4, 7, and 9 are 510 d, 46 h, and <30 min, respectively.
Chemical Properties
Trichlorfon is a white to pale yellow crystalline solid.
Uses
Trichlorfon is an irreversible organophosophate acetylcholinesterase inhibitor and the prodrug of Dichlorvos (D435950). Trichlorfon have also shown potential actions to be utilized as an effective org anophosphorus pesticide.
Indications
Metrifonate is an organophosphorous compound that is effective only in the treatment of S. haematobium. The active metabolite, dichlorvos, inactivates acetylcholinesterase and potentiates inhibitory cholinergic effects. The schistosomes are swept away from the bladder to the lungs and are trapped. Therapeutic doses produce no untoward side effects except for mild cholinergic symptoms. It is contraindicated in pregnancy, previous insecticide exposure, or with depolarizing neuromuscular blockers. Metrifonate is not available in the United States.
Definition
ChEBI: A phosphonic ester that is dimethyl phosphonate in which the hydrogen atom attched to the phosphorous is substituted by a 2,2,2-trichloro-1-hydroxyethyl group.
Antimicrobial activity
Useful activity is restricted to Schistosoma haematobium. It has little activity against other schistosomes. Although it exhibits activity against several other helminths, it is not used for their treatment.
General Description
Chlorophos is a white crystalline solid. Soluble in water, benzene, chloroform, ether; insoluble in oils. Chlorophos is a wettable powder. Chlorophos can cause illness by inhalation, skin absorption and/or ingestion. Chlorophos is used as a pesticide.
Air & Water Reactions
Chlorophos decomposes at higher temperatures in water and at pH <5.5. Chlorophos is sensitive to prolonged exposure to moisture. Chlorophos is unstable in alkaline solutions.
Reactivity Profile
Chlorophos is incompatible with alkalis. Chlorophos is corrosive to black iron and mild steel. Chlorophos is corrosive to metals. Chlorophos is subject to hydrolysis.
Health Hazard
INHALATION, INGESTION, AND SKIN ABSORPTION. Inhibits cholinesterase. Headache, depressed appetite, nausea, miosis are symptoms of light exposures. Moderate effects are peritoneal paralysis, diarrhea, salivation, lacrimation, sweating, dyspnea, substernal tightness, slow pulse, tremors, muscular cramps and ataxia. Severe symptoms are: pyrexia, cyanosis, pulmonary edema, areflexia, loss of sphincter control, paralysis, coma, heart block, shock and respiratory failure. EYES: Increases permeability of blood vessels in anterior eye. Reduces corneal sensitivity with glaucoma, abnormalities in intraocular tension or decreased visual acuity.
Fire Hazard
Combustible material: may burn but does not ignite readily. Containers may explode when heated. Runoff may pollute waterways. Substance may be transported in a molten form.
Pharmaceutical Applications
An organophosphorus compound. It is soluble in water and stable at room temperature. At higher temperatures it decomposes to the insecticide dichlorvos.
Trade name
AEROL 1 (PESTICIDE)®; AGROFOROTOX®; ANTHON®; BAY 15922®; BAYER 15922®; BAYER L 13/59®; BILARCIL®; BOVINOX®[C]; BRITON®; BRITTEN®; CEKUFON®; CHLORAK®; CHLOROFTALM®; CICLO-SOM®; COMBOT®; COMBOT EQUINE®; DANEX®[C]; DEP®; DEPTHON®; DIMETOX®; DIPTEREX®; DIPTEREX® 50; DIPTEVU®; DITRIFON®; DYLOX®; DYLOX-METASYSTOX-R®; DYREX®; DYVON®; EQUINO-ACID®; EQUINO-AID®; FLIBOL E®; FLIEGENTELLE®; FOROTOX®; FOSCHLOR®; FOSCHLOR R®; FOSCHLOR R-50®; LEIVASOM®; LOISOL®; MASOTEN®[C]; MAZOTEN®; NEGUVON®; NEGUVON A®; PHOSCHLOR R50®; PROXOL®; RICIFON®; RITSIFON®; SATOX 20WSC®; SOLDEP®; SOTIPOX®; TRICHLORPHON FN®; TRINEX®; TUGON®; TUGON FLY BAIT®; TUGON STABLE SPRAY®; VERMICIDE BAYER 2349®; VOLFARTOL®; VOTEXIT®; WEC 50®; WOTEXIT®
Pharmacokinetics
Metrifonate is rapidly absorbed after oral administration, achieving a peak concentration in plasma within 1–2 h. It undergoes chemical transformation to dichlorvos, which is the active molecule. Dichlorvos is rapidly and extensively metabolized and excreted mainly in the urine.
Clinical Use
Urinary schistosomiasis (especially mass chemotherapy control programs)
Side effects
Various side effects such as abdominal pain, gastrointestinal upsets and vertigo occur in many patients. As the worms release their hold of the veins in the bladder they pass through the blood system to the lungs, where they disintegrate; this may cause some of the side effects. Cholinesterase levels in the blood and on erythrocytes are depressed, but the significance of this is unknown.
Safety Profile
Poison by ingestion, inhalation, inti-aperitoneal, subcutaneous, intravenous, and intramuscular routes. Moderately toxic by skin contact. Human systemic effects: true cholinesterase. Experimental teratogenic and reproductive effects. Questionable carcinogen with experimental carcinogenic and tumorigenic data. Human mutation data reported. An eye irritant. When heated to decomposition it emits very toxic fumes of Cland POx.
Potential Exposure
Trichlorfon is used as an agricultural and forest insecticide.
Carcinogenicity
When rats were fed diets that contained 0, 50, 100, 200, 250, 400, 500, or 1000 ppm (equivalent to about 0.5, 12.5, 25, or 50 mg/kg/day) for 17 or 24 months, no treatment-related effects occurred in those fed 50–250 ppm . Histopathological results suggested the occurrence of mammary tumors in rats fed 400, 500, and 1000 ppm. In another study, when rats were fed diets containing 0, 50, 250, 500, or 1000 ppm (equivalent to about 2.5, 12.5, 25, or 50 mg/kg/day) trichlorfon for 24 months, no treatment-related effects other than whole-blood cholinesterase depression at 1000 ppm occurred . There was no increase in the incidence of either benign or malignant tumors, including mammary tumors.
Environmental Fate
Soil. Trichlorfon degraded in soil to dichlorvos (alkaline conditions) and desmethyl
dichlorvos (Mattson et al., 1955).
Plant. In cotton leaves, the metabolites identified included dichlorvos, phosphoric acid,
O-demethyl dichlorvos, O-demethyl trichlorfon, methyl phosphate and dimethyl phosphate
(Bull and Ridgway, 1969). Chloral hydrate and trichloroethanol were r
Pieper and Richmond (1976) studied the persistence of trichlorfon in various foliage
following an application rate of 1.13 kg/ha. Concentrations of the insecticide found at day
0 and 14 were 81.7 ppm and 7 ppb for willow foliage, 12.6 ppm and 670 ppb for
Chemical/Physical. At 100°C, trichlorfon decomposes to chloral. Decomposed by hot
water at pH <5 forming dichlorvos (Worthing and Hance, 1991).
Metabolism
Trichlorfon administered to mammals is rapidly metabolized and excreted almost completely in the urine within 6 h. Majormetabolites are dimethyl hydrogen phosphate, methyl dihydrogen phosphate, and conjugates of dichloroacetic acid and trichloroethanol. Trichlorfon is rapidly broken down in soil.
Shipping
UN2783 Organophosphorus pesticides, solid, toxic, Hazard Class: 6.1; Labels: 6.1-Poisonous materials.
Incompatibilities
This chemical may be characterized as an organo-phosphate or-chlorine compound. Organophosphates are susceptible to formation of highly toxic and flammable phosphine gas in the presence of strong reducin g agents such as hydrideds and active metals. Partial oxidation by oxidizing agents may result in the release of toxic phosphorus oxides.Alkaline materials: lime, lime sulfur, etc. Corrosive to iron, steel and possibly to other metals.
Waste Disposal
Add a combustible solvent and burn in a furnace equipped with an afterburner and an alkali scrubber.In accordance with 40CFR165, follow recommendations for the disposal of pesticides and pesticide containers. Must be disposed properly by following package label directions or by contacting your local or federal environmental control agency, or by contacting your regional EPA office.
Trichlorfon Preparation Products And Raw materials
Raw materials
1of2
chevron_rightPreparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Pharmaffiliates Analytics and Synthetics P. Ltd | +91-172-5066494 | Haryana, India | 6773 | 58 | Inquiry |
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6351 | 58 | Inquiry |
| Hebei Mojin Biotechnology Co., Ltd | +86 13288715578 +8613288715578 | China | 12584 | 58 | Inquiry |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | China | 21662 | 55 | Inquiry |
| career henan chemical co | +86-0371-86658258 +8613203830695 | China | 29899 | 58 | Inquiry |
| SHANDONG ZHI SHANG CHEMICAL CO.LTD | +86 18953170293 | China | 2931 | 58 | Inquiry |
| Hebei Guanlang Biotechnology Co., Ltd. | +86-19930503282 | China | 8818 | 58 | Inquiry |
| Chongqing Chemdad Co., Ltd | +86-023-6139-8061 +86-86-13650506873 | China | 39916 | 58 | Inquiry |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | China | 49403 | 58 | Inquiry |
| Hubei Ipure Biology Co., Ltd | +8613367258412 | China | 10326 | 58 | Inquiry |
52-68-6(Trichlorfon)Related Search:
1of4
chevron_right




