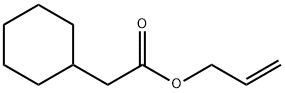
ALLYLCYCLOHEXANE ACETATE
- CAS No.
- 4728-82-9
- Chemical Name:
- ALLYLCYCLOHEXANE ACETATE
- Synonyms
- ALLYLCYCLOHEXYLACETATE;ALLYLCYCLOHEXANE ACETATE;Cyclohexylacetic acid allyl ester;ALLYLCYCLOHEXANE ACETATE USP/EP/BP;Cyclohexaneacetic acid, 2-propenyl ester;Cyclohexaneacetic acid, 2-propen-1-yl ester
- CBNumber:
- CB7477580
- Molecular Formula:
- C11H18O2
- Molecular Weight:
- 182.26
- MOL File:
- 4728-82-9.mol
- Modify Date:
- 2024/6/5 9:38:15
| Boiling point | 275.67°C (rough estimate) |
|---|---|
| Density | 0.9813 (rough estimate) |
| refractive index | 1.4614 (estimate) |
| FEMA | 2023 | ALLYL CYCLOHEXANEACETATE |
| color | A colourless liquid. |
| Odor | at 100.00 %. sweet ripe fruity pineapple green peach apricot |
| Odor Type | fruity |
| JECFA Number | 12 |
| LogP | 3.59 |
| EPA Substance Registry System | Cyclohexaneacetic acid, 2-propenyl ester (4728-82-9) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H315-H312-H302-H319 |
| Precautionary statements | P264-P270-P301+P312-P330-P501-P280-P302+P352-P312-P322-P363-P501-P264-P280-P302+P352-P321-P332+P313-P362-P264-P280-P305+P351+P338-P337+P313P |
| Toxicity | The acute oral LD50 value in rats was reported as 0.90 g/kg (0.56-1.24 g/kg) (Moreno, 1974) and the acute dermal LD50 value in rabbits as 1.25 g/kg (0.35-2.15 g/kg) (Moreno, 1974). |
ALLYLCYCLOHEXANE ACETATE Chemical Properties,Uses,Production
Chemical Properties
Colorless liquid, almost insoluble in water, miscible with alcohol, essential oils and flavor chemicals, perfume materials, etc. Pronounced “mixed-fruity“ odor, sweet, lasting and less ethereal than the lower aliphatic acetates. The flavor is overall fruity (“tutti-frutti”) with some resemblance to Pineapple, Peach and Apricot.
Occurrence
Has apparently not been reported to occur in nature.
Preparation
By esterfication of cyclohexane, acetic acid and allyl alcohol in the presence of benzene
Metabolism
The hydrolysis of ester linkages in foreign compounds may be catalysed by many different esterases; most of these have a low degree of substrate specificity and they are to be found in all animals and bacteria (Parke, 1968). In the rat, allyl acetate and allyl alcohol are metabolized to 3-hydroxypropylmercapturic acid, which is excreted in the urine (Clapp, Kaye & Young, 1969). In dogs, cyclohexylacetic acid was not aromatized and was probably completely oxidized in the body by β-oxidation (Bernhard, 1937; Williams, 1959).
ALLYLCYCLOHEXANE ACETATE Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Dideu Industries Group Limited | +86-29-89586680 +86-15129568250 | China | 26165 | 58 | Inquiry |
| ZHEJIANG JIUZHOU CHEM CO., LTD | +86-0576225566889 +86-13454675544 | China | 19947 | 58 | Inquiry |
| BOC Sciences | 1-631-485-4226; 16314854226 | United States | 14059 | 65 | Inquiry |
| Baoji Didu Pharmaceutical and Chemical Co., Ltd | 029-61856358 15829046862 | China | 10011 | 58 | Inquiry |
| TCI (Shanghai) Chemical Trading Co., Ltd. | 021-021-61109150 | China | 31163 | 58 | Inquiry |
| ChemSampCo, Inc. | 609 656 2440 | United States | 3585 | 60 | Inquiry |
| 3B Scientific Corporation | 847.281.9822 | United States | 6744 | 47 | Inquiry |
| Shanghai wencai New Material Technology Co., Ltd. | 15721586846 | China | 2796 | 58 | Inquiry |
| Jiangyin Healthway International Trade Co., Ltd | 86-510-86023169 | United States | 1006 | 58 | Inquiry |
| Pyrazine Specialties, Inc. | (404) 524-6744 | United States | 1265 | 30 | Inquiry |
4728-82-9(ALLYLCYCLOHEXANE ACETATE)Related Search:
1of2
chevron_right




