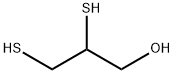
2,3-Dimercapto-1-propanol
- CAS No.
- 59-52-9
- Chemical Name:
- 2,3-Dimercapto-1-propanol
- Synonyms
- BAL;DIMERCAPROL;2,3-DIMERCAPTOPROPAN-1-OL;BACS;BAL1;Parp9;Panobal;Antoxol;Dimersol;usafme-1
- CBNumber:
- CB7684878
- Molecular Formula:
- C3H8OS2
- Molecular Weight:
- 124.22
- MOL File:
- 59-52-9.mol
- MSDS File:
- SDS
- Modify Date:
- 2023/9/7 16:59:03
| Melting point | 77 °C |
|---|---|
| Boiling point | 120 °C15 mm Hg(lit.) |
| Density | 1.239 g/mL at 25 °C(lit.) |
| vapor density | 4.3 |
| vapor pressure | 7.4 hPa (100 °C) |
| refractive index |
n |
| Flash point | >230 °F |
| storage temp. | Store at +2°C to +8°C. |
| solubility | 87g/l (slow decomposition) |
| pka | 9.00±0.25(Predicted) |
| form | Liquid |
| color | Clear colorless to slightly yellow |
| PH | 5.0-6.5 (H2O, 20℃)(saturated solution) |
| Odor | pungent odor |
| Water Solubility | 87 g/L (25 ºC) |
| Sensitive | Air Sensitive |
| Merck | 14,3209 |
| BRN | 1732058 |
| Stability | Stable. Combustible. Incompatible with strong oxidizing agents, many metals. |
| CAS DataBase Reference | 59-52-9(CAS DataBase Reference) |
| NIST Chemistry Reference | Dimercaprol(59-52-9) |
| EPA Substance Registry System | 2,3-Dimercaptopropanol (59-52-9) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS06 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H301-H315-H319-H335 | |||||||||
| Precautionary statements | P301+P310+P330-P302+P352-P305+P351+P338 | |||||||||
| Hazard Codes | Xn,Xi | |||||||||
| Risk Statements | 22-36/37/38-36/38 | |||||||||
| Safety Statements | 26-36-24/25-23 | |||||||||
| RIDADR | UN 2810 6.1/PG 3 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | UB2625000 | |||||||||
| F | 8-9-13-23 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 6.1 | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 29309070 | |||||||||
| Toxicity | LD50 i.m. in rats: 86.7 mg/kg (Zvirblis, Ellin) | |||||||||
| NFPA 704 |
|
2,3-Dimercapto-1-propanol price More Price(8)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | 64046 | 2,3-Dimercapto-1-propanol ≥98% (iodometric) | 59-52-9 | 10ML | ₹19116.95 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | SRP0197 | PARP9 Active human recombinant, expressed in baculovirus infected insect cells, ≥80% (SDS-PAGE) | 59-52-9 | 10μG | ₹71783.7 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 64046 | 2,3-Dimercapto-1-propanol ≥98% (iodometric) | 59-52-9 | 6X10ML | ₹82843.73 | 2022-06-14 | Buy |
| TCI Chemicals (India) | D0621 | 2,3-Dimercapto-1-propanol min. 95.0 % | 59-52-9 | 1G | ₹2900 | 2022-05-26 | Buy |
| TCI Chemicals (India) | D0621 | 2,3-Dimercapto-1-propanol min. 95.0 % | 59-52-9 | 5G | ₹9300 | 2022-05-26 | Buy |
2,3-Dimercapto-1-propanol Chemical Properties,Uses,Production
Description
Dimercaprol (INN) or British anti - Lewisite (abbreviated BAL), is a compound developed by British biochemists at Oxford University during World War II . It was developed secretly as an antidote for lewisite, the now - obsolete arsenic - based chemical warfare agent . Today, it is used medically in treatment of arsenic, mercury , gold, lead, antimony, and other toxic metal poisoning . In addition , it has in the past been used for the treatment of Wilson's disease, a genetic disorder in which the body tends to retain copper.
Chemical Properties
colourless oily liquid with a typically offensive mercaptan smell
Uses
2,3-Dimercapto-1-propanol has been used in synthesizing novel (2-substituted phenyl-1,3-dithiolan-4-yl) methanol (PDTM) derivatives, which are potent tyrosinase inhibitors. It can also be considered for developing new drugs against AIDS due to its ability to inhibit HIV-1 tat activity.
Definition
ChEBI: A dithiol that is propane-1,2-dithiol in which one of the methyl hydrogens is replaced by a hydroxy group. a chelating agent originally developed during World War II as an experimental antidote against the arsenic-based poison gas Lewisite, it has been use clinically since 1949 for the treatment of poisoning by arsenic, mercury and gold. It can also be used for treatment of poisoning by antimony, bismuth and possibly thallium, and (with sodium calcium edetate) in cases of acute leaad poisoning. Administrati n is by (painful) intramuscular injection of a suspension of dimercaprol in peanut oil, typically every 4 hours for 2-10 days depending on the toxicity. In the past, dimercaprol was also used for the treatment of Wilson's disease, a severely debilitating g netic disorder in which the body tends to retain copper, with resultant liver and brain injury.
Biological Functions
Arsenic and some other heavy metals act by chemically reacting with adjacent thiol residues on metabolic enzymes, creating a chelate complex that inhibits the affected enzyme's activity. Dimercaprol competes with the thiol groups for binding the metal ion, which is then excreted in the urine .
Dimercaprol is itself toxic, with a narrow therapeutic range and a tendency to concentrate arsenic in some organs. Other drawbacks include the need to administer it by painful intramuscular injection. Serious side effects include nephrotoxicity and hypertension.
Dimercaprol has been found to form stable chelates in vivo with many other toxic metals including inorganic mercury, antimony, bismuth, cadmium, chromium, cobalt, gold, and nickel. However, it is not necessarily the treatment of choice for toxicity to these metals. Dimercaprol has been used as an adjunct in the treatment of the acute encephalopathy of lead toxicity. It is a potentially toxic drug, and its use may be accompanied by multiple side effects. Although treatment with dimercaprol will increase the excretion of cadmium, there is a concomitant increase in renal cadmium concentration, so that its use in case of cadmium toxicity is to be avoided. It does, however, remove inorganic mercury from the kidneys; but is not useful in the treatment of alkylmercury or phenyl mercury toxicity. Dimercaprol also enhances the toxicity of selenium and tellurium, so it is not to be used to remove these elements from the body.
General Description
Clear colorless viscous liquid with a pungent offensive odor of mercaptans. Used as a medicine and an antidote to the chemical warfare agent LEWISITE.
Air & Water Reactions
Moderately soluble in water with decomposition [Hawley].
Reactivity Profile
2,3-Dimercapto-1-propanol forms highly stable chelates with a variety of metal ions. Organosulfides are incompatible with acids, diazo and azo compounds, halocarbons, isocyanates, aldehydes, alkali metals, nitrides, hydrides, and other strong reducing agents. Reactions with these materials generate heat and in many cases hydrogen gas. Many of these compounds may liberate hydrogen sulfide upon decomposition or reaction with an acid.
Fire Hazard
2,3-Dimercapto-1-propanol is probably combustible.
Pharmaceutical Applications
Dimercaprol (BAL) is a chelating agent used as an antidote for arsenic, antimony, bismuth, gold and mercury poisoning. It has the chemical name 2,3-dimercapto-1-propanol and is a clear, colourless or slightly yellow liquid.
Clinical Use
2,3-Dimercapto-1-propanol, BAL, or dithioglycerol is afoul-smelling, colorless liquid. It is soluble in water (1:20)and alcohol. It was developed by the British during WorldWar II as an antidote for “Lewisite,” hence the name Britishanti-Lewisite or BAL. Dimercaprol is effective topicallyand systematically as an antidote for poisoning caused byarsenic, antimony, mercury, gold, and lead. It can, therefore,also be used to treat arsenic and antimony toxicity associatedwith overdose or accidental ingestion of organoarsenicalsor organoantimonials.The antidotal properties of BAL are associated with theproperty of heavy metals to react with sulfhydryl (SH)groups in proteins (e.g., the enzyme pyruvate oxidase) andinterfere with their normal function. 1,2-Dithiol compoundssuch as BAL compete effectively with such proteins for themetal by reversibly forming metal ring compounds.These are relatively nontoxic, metabolically conjugated(as glucuronides), and rapidly excreted.BAL may be applied topically as an ointment or injectedintramuscularly as a 5% or 10% solution in peanut oil.
Safety Profile
Poison via ingestion, intramuscular, parenteral, intraperitoneal, and intravenous routes. Experimental teratogenic effects. Human systemic effects by intramuscular route: hemorrhage and dermatitis. Human blood and systemic skin effects by intramuscular route. It causes redness and swelling when applied locally to the skin, but does not produce blisters or ulcers. Intensely irritating to eyes and mucous membranes. Systemic symptoms are caused by injection. When heated to decomposition, it emits toxic fumes of SO,. Used as an antidote to arsenic, gold, and mercury poisoning.
Environmental Fate
BAL is believed to compete with tissue sulfhydryl groups and interferes with cellular respiration. It also competes with metallic cofactors of metabolic enzyme systems and increases capillary permeability. Metabolic degradation and excretion are essentially complete within 4 h. BAL not excreted as dimercaprol– metal complex is quickly metabolized by the liver and excreted as an inactive product in the urine. Because it is a lipophilic drug, it penetrates rapidly the intracellular spaces. The highest concentrations are found in the liver, kidneys, brain, and small intestine. Due to its lipophilic characteristic, the complexes formed with mercury and other metals may be redistributed into sensitive cells in the brain following dimercaprol treatment.
Purification Methods
Precipitate BAL as the Hg mercaptide [see Bj.berg Chem Ber 75 13 1942], regenerate with H2S, and distil it under a vacuum [Rosenblatt & Jean Anal Chem 951 1955]. It is an antidote for heavy metal (As, Hg, Au etc) poisoning. [Beilstein 1 IV 2770.]
2,3-Dimercapto-1-propanol Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| SPD CHEMICALS | +91-7075800742 +91-7075800742 | Hyderabad, India | 214 | 58 | Inquiry |
| VDL ORGANICS PVT LTD | +91-9246335727 +91-8328068806 | Telangana, India | 50 | 58 | Inquiry |
| CHEMAZON LABORATORIES | +91 9848551964 | Hyderabad, India | 1320 | 58 | Inquiry |
| Alfa Aesar | 1 800 209 7001 | Maharashtra, India | 6913 | 58 | Inquiry |
| Clearsynth Labs | 91-22-45045900 | Maharashtra, India | 3889 | 58 | Inquiry |
| Pharma Affiliates | 172-5066494 | Haryana, India | 6761 | 58 | Inquiry |
| Pharmaffiliates Analytics & Synthetics (P) Ltd. | 91-172-5066494 | Haryana, India | 631 | 58 | Inquiry |
| Manus Aktteva Biopharma LLP | 08048250218Ext 800 | Ahmedabad, India | 655 | 58 | Inquiry |
| Samex Overseas | 08068441146Ext 740 | Gujarat, India | 124 | 58 | Inquiry |
| ALTAIR ALCHEMY Laboratories | 91--9014276848 | Hyderabad, India | 324 | 58 | Inquiry |
| Supplier | Advantage |
|---|---|
| SPD CHEMICALS | 58 |
| VDL ORGANICS PVT LTD | 58 |
| CHEMAZON LABORATORIES | 58 |
| Alfa Aesar | 58 |
| Clearsynth Labs | 58 |
| Pharma Affiliates | 58 |
| Pharmaffiliates Analytics & Synthetics (P) Ltd. | 58 |
| Manus Aktteva Biopharma LLP | 58 |
| Samex Overseas | 58 |
| ALTAIR ALCHEMY Laboratories | 58 |
Related articles
- Uses of 2,3-Dimercapto-1-propanol
- British Anti-Lewisite (BAL) is a synthetic therapeutic substance developed during World War II for using as an antidote agains....
- Nov 16,2021
59-52-9(2,3-Dimercapto-1-propanol)Related Search:
1of4
chevron_right




