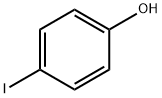
4-Iodophenol
- CAS No.
- 540-38-5
- Chemical Name:
- 4-Iodophenol
- Synonyms
- P-IODOPHENOL;p-Jodphenol;p-iodo-pheno;4-IODOPHENOL;4-iodo-pheno;4-lodophenoI;Phenol, p-iodo-;PHENOL, 4-IODO-;4-Iodophenol99%;PARA-IODOPHENOL
- CBNumber:
- CB8328252
- Molecular Formula:
- C6H5IO
- Molecular Weight:
- 220.01
- MOL File:
- 540-38-5.mol
- MSDS File:
- SDS
- Modify Date:
- 2023/12/24 16:54:11
| Melting point | 92-94 °C(lit.) |
|---|---|
| Boiling point | 138 °C5 mm Hg(lit.) |
| Density | 1.8573 |
| Flash point | 138°C/5mm |
| storage temp. | Refrigerator |
| solubility | Chloroform (Slightly), Methanol (Slightly) |
| form | Crystals or Crystalline Powder |
| pka | 9.33(at 25℃) |
| color | Pink or beige to brown |
| Water Solubility | slightly soluble |
| Sensitive | Light Sensitive |
| Merck | 14,5036 |
| BRN | 1904544 |
| Stability | Stable. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 540-38-5(CAS DataBase Reference) |
| NIST Chemistry Reference | Phenol, 4-iodo-(540-38-5) |
| EPA Substance Registry System | p-Iodophenol (540-38-5) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS05,GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H302+H312-H314 | |||||||||
| Precautionary statements | P260-P270-P280-P301+P312-P303+P361+P353-P305+P351+P338 | |||||||||
| Hazard Codes | C,Xi,Xn | |||||||||
| Risk Statements | 21/22-34-36/37/38-20/21/22 | |||||||||
| Safety Statements | 26-36/37/39-45-22 | |||||||||
| RIDADR | UN 3261 8/PG 3 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | SL5600000 | |||||||||
| Hazard Note | Irritant | |||||||||
| TSCA | T | |||||||||
| HazardClass | IRRITANT, LACHRYMATOR | |||||||||
| HS Code | 29081900 | |||||||||
| NFPA 704 |
|
4-Iodophenol price More Price(11)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | I10201 | 4-Iodophenol 99% | 540-38-5 | 10G | ₹5748.08 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | I10201 | 4-Iodophenol 99% | 540-38-5 | 25G | ₹7913.08 | 2022-06-14 | Buy |
| TCI Chemicals (India) | I0840 | 4-Iodophenol [for Biochemical Research] min. 98.0 % | 540-38-5 | 1G | ₹2700 | 2022-05-26 | Buy |
| TCI Chemicals (India) | I0840 | 4-Iodophenol [for Biochemical Research] min. 98.0 % | 540-38-5 | 5G | ₹4500 | 2022-05-26 | Buy |
| TCI Chemicals (India) | I0311 | 4-Iodophenol min. 98.0 % | 540-38-5 | 10G | ₹3100 | 2022-05-26 | Buy |
4-Iodophenol Chemical Properties,Uses,Production
Chemical Properties
Light brown powder. It dissolves slightly in water, but readily dissolves in ethanol, ether, and other organic solvents.
Uses
4-Iodophenol is used in chemiluminescence imaging assays and experiments, as diagnostic agents in order to detect cancer cells in patients. It is also utilized in synthesizing agonists for the estrogen β receptor. Additionally, The compound finds extensive applications in various medical industries as well as in human and animal nutrition products such as pharmaceutical intermediates, and polarizing films for Liquid Crystal Display (LCD) chemicals.
Preparation
It is obtained from 4-Aminophenol by diazotisation and substitution.
Application
4-Iodophenol can be used as a building block for the synthesis of:
Hydroxybiaryls by reacting with aryl boronic acids via Pd-catalyzed Suzuki-Miyaura coupling reaction.[1][2]
Aryl substituted olefins by reacting with acrylates via Pd-catalyzed Heck reaction.[3]
Iodinated-4-aryloxymethylcoumarins [4]
Hydroxylated stilbenoids [5] and psammaplysenes A and B.[6]
Metabolism
4-Iodophenol has known human metabolites that include (2S,3S,4S,5R)-3,4,5-trihydroxy-6-(4-iodophenoxy)oxane-2-carboxylic acid.
Purification Methods
Crystallise 4-iodophenol from pet ether (b 80-100o) or distil it in vacuo. If the material has a brown or violet color, then dissolve it in CHCl3, shake it with 5% sodium thiosulfate solution until is colourless. Dry (Na2SO4), extract, evaporate and disil the residue in vacuo. [Dains & Eberly Org Synth Coll Vol II 355 1948, Beilstein 6 IV 1074.]
References
[1] HIDEHIRO SAKURAI Toshikazu H Tatsuya Tsukuda. Pd/C as a Reusable Catalyst for the Coupling Reaction of Halophenols and Arylboronic Acids in Aqueous Media[J]. The Journal of Organic Chemistry, 2002, 67 8: 2721-2722. DOI:10.1021/jo016342k.
[2] PIOTR WAWRZYNIAK Joachim H. Microwave-Promoted Suzuki—Miyaura Coupling of Arylboronic Acids with 1-Bromo-2-naphthol, o-Bromophenol, and o-Chlorophenol.[J]. ChemInform, 2007, 38 14. DOI:10.1002/chin.200714105.
[3] LUNXIANG YIN Juergen L. Carbon—Carbon Coupling Reactions Catalyzed by Heterogeneous Palladium Catalysts[J]. ChemInform, 2007, 38 22. DOI:10.1002/chin.200722237.
[4] MAHANTESHA BASANAGOUDA . Synthesis, structure–activity relationship of iodinated-4-aryloxymethyl-coumarins as potential anti-cancer and anti-mycobacterial agents[J]. European Journal of Medicinal Chemistry, 2014, 74: Pages 225-233. DOI:10.1016/j.ejmech.2013.12.061.
[5] AMIT SHARD. Tandem Heck/Decarboxylation/Heck Strategy: Protecting-Group-Free Synthesis of Symmetric and Unsymmetric Hydroxylated Stilbenoids?[J]. Angewandte Chemie International Edition, 2012, 51 49: 12250-12253. DOI:10.1002/anie.201206346.
[6] SAVVAS N. GEORGIADES Jon C. Total Synthesis of Psammaplysenes A and B, Naturally Occurring Inhibitors of FOXO1a Nuclear Export[J]. Organic Letters, 2005, 7 19: 4091-4094. DOI:10.1021/ol0513286.
4-Iodophenol Preparation Products And Raw materials
Raw materials
Preparation Products
1of3
chevron_right| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| GLR Innovations | +91 9891111994 | New Delhi, India | 4542 | 58 | Inquiry |
| PEE ESS AROMATICS | +919382160846 | Tamil Nadu, India | 53 | 58 | Inquiry |
| SYNIX LABS | +919849178742 | Hyderabad, India | 108 | 58 | Inquiry |
| Fluoro chem Pvt.Ltd | +91 8149270195 | Maharashtra, India | 35 | 58 | Inquiry |
| Dynarx Technology India Limited | +91-9960099480 +91-9960099480 | Bangalore, India | 71 | 58 | Inquiry |
| REDDY N REDDY PHARMACEUTICALS | +91-9848096759 +91-9848096759 | Hyderabad, India | 1989 | 58 | Inquiry |
| Cefa-Cilinas Biotics Pvt Ltd | +91-7875033155 +91-8080701561 | Maharashtra, India | 121 | 58 | Inquiry |
| JSK Chemicals | +919879767970 | Gujarat, India | 3756 | 58 | Inquiry |
| AVIGYABIOSCIENCES PRIVATE LIMITED | +91-9849533087 +91-9849533087 | Telangana, India | 156 | 58 | Inquiry |
| Dr. ASCRO Bio Sciences Private Limited | +undefined-7382260502 +undefined-+91 9182680914 | Telangana, India | 51 | 58 | Inquiry |
540-38-5(4-Iodophenol)Related Search:
1of4
chevron_right




