POTASSIUM HYPOCHLORITE
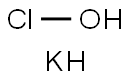
- CAS No.
- 7778-66-7
- Chemical Name:
- POTASSIUM HYPOCHLORITE
- Synonyms
- potassiumchlorideoxide;POTASSIUM HYPOCHLORITE;potassiumhypochloritesolution;Hypochlorousacid,potassiumsalt;Hypochlorous acid, potassium salt (1:1)
- CBNumber:
- CB8332697
- Molecular Formula:
- ClH2KO
- Molecular Weight:
- 92.56
- MOL File:
- 7778-66-7.mol
- Modify Date:
- 2023/10/17 17:11:49
| storage temp. | 0-6°C |
|---|---|
| color | exists only in aqueous solution |
| EPA Substance Registry System | Potassium hypochlorite (7778-66-7) |
POTASSIUM HYPOCHLORITE Chemical Properties,Uses,Production
General Description
Also known as eau de Javelle, KOCI is colorless aqueous solution with a pungent irritating chlorine odor. It is formed by passing chlorine through a solution of potassium hydroxide. The aqueous solution is strongly oxidizing,and is used as a bleach.
Air & Water Reactions
Soluble in water.
Reactivity Profile
POTASSIUM HYPOCHLORITE is a powerful oxidizing agent. May form highly explosive NCl3 on contact with urea. Heating or contact with acids produces highly toxic fumes of chlorine gas [Sax, 9th ed., 1996, p. 1905]. May react vigorously with carbon; reacts potentially explosively with finely divided carbon. Reacts with acetylene to form explosive chloroacetylenes. Reactions with organic matter, oil, hydrocarbons; alcohols may lead to explosions. Reactions with nitromethane, methanol, ethanol (and other alcohols) can become violent after a delay. Reacts with possible ignition and/or explosion with organic sulfur compounds and with sulfides. Decomposes evolving oxygen, a change that can be catalyzed by rust on metal containers. Forms highly explosive NCl3 on contact with urea or ammonia. Evolves highly toxic gaseous chlorine gas when heated or on contact with acids [Sax, 9th ed., 1996, p. 1905]. A mixture with damp sulfur reacted violently, and molten sulfur was ejected [Chem Eng. News, 1965, 46(29), 6]. The combination of calcium hypochlorite, sodium hydrogen sulfate, starch, and sodium carbonate, when compressed, caused the materials to incandescence, followed by explosion, [Ind. Eng. Chem., 1937, 15, 282].
Safety Profile
A poison by all routes. Powerful irritant and corrosive to skin, eyes, and mucous membranes. Questionable carcinogen. When heated to decomposition it emits toxic fumes of K2O and Cl-. See also HYPOCHLORITES.
POTASSIUM HYPOCHLORITE Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Triveni chemicals | 08048762458 | New Delhi, India | 6093 | 58 | Inquiry |
| Chongqing Chemdad Co., Ltd | +86-023-6139-8061 +86-86-13650506873 | China | 39916 | 58 | Inquiry |
| Shanghai ChuCheng Trade Co., Ltd. | 021-5983532 15201998618 | China | 4619 | 58 | Inquiry |
| Shaanxi DIDU pharmaceutical and Chemical Co., Ltd | 17691182729 18161915376 | China | 10011 | 58 | Inquiry |
| Kanto Chemical Co., Inc. | +81 3 3663 7631 | Japan | 6762 | 74 | Inquiry |
| Shanghai Trustin Chemical Co., Ltd | 021-61086350 | China | 5364 | 51 | Inquiry |
| Birko Corporation | 800.525.0476 | United States | 51 | 66 | Inquiry |
| CHEMICAL LAND21 | 82- 2 -783 - 8063 | South Korea | 6315 | 74 | Inquiry |
| Cangzhou Fengmao Biotech Co., Ltd. | 0317-5304268 13582748294 | China | 112 | 60 | Inquiry |
| Alfa Chemistry | +1 (201) 478-8534 | United States | 6824 | 0 | Inquiry |
7778-66-7(POTASSIUM HYPOCHLORITE)Related Search:
1of4
chevron_right




