POTASSIUM NITRITE
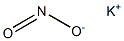
- CAS No.
- 7758-09-0
- Chemical Name:
- POTASSIUM NITRITE
- Synonyms
- Kaliumnitrit;NPotassiuM nitri;POTASSIUM NITRITE;PotassiumNitriteFcc;Potassium nitrite,AR;potassiumnitrite(1:1);POTASSIUM NITRITE XTL;Nitrous acid potassium;POTASSIUM NITRITE, ACS;POTASSIUM NITRITE R. G.
- CBNumber:
- CB8694434
- Molecular Formula:
- KNO2
- Molecular Weight:
- 85.1038
- MOL File:
- 7758-09-0.mol
- MSDS File:
- SDS
- Modify Date:
- 2023/6/28 13:45:06
| Melting point | 350 °C (dec.)(lit.) |
|---|---|
| Boiling point | explodes at 537℃ [HAW93] |
| Density | 1,92 g/cm3 |
| vapor density | 2.9 (vs air) |
| storage temp. | Store at +5°C to +30°C. |
| solubility | water: soluble0.35 part |
| form | Solid |
| Specific Gravity | 1.915 |
| color | White to slightly yellow |
| PH | 7-10 (50g/l, H2O, 20℃) |
| Odor | Odorless |
| PH Range | 7 - 10 |
| Water Solubility | Soluble in water, hot alcohol, liquid NH{3}Soluble in water, alcohol and ammonia. |
| Sensitive | Hygroscopic |
| Merck | 14,7649 |
| Stability | Stability Stable, but strong oxidizer - contact with combustible material may lead to fire. Incompatible with strong reducing agents, strong acids, combustible materials, cyanides, ammonium salts. |
| CAS DataBase Reference | 7758-09-0(CAS DataBase Reference) |
| EPA Substance Registry System | Potassium nitrite (7758-09-0) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS03,GHS06,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H272-H301-H319-H400 | |||||||||
| Precautionary statements | P210-P220-P264-P273-P301+P310-P305+P351+P338 | |||||||||
| Hazard Codes | O,T,N | |||||||||
| Risk Statements | 8-25-50 | |||||||||
| Safety Statements | 45-61 | |||||||||
| RIDADR | UN 1488 5.1/PG 2 | |||||||||
| WGK Germany | 2 | |||||||||
| RTECS | TT3750000 | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 2834 10 00 | |||||||||
| HazardClass | 5.1 | |||||||||
| PackingGroup | II | |||||||||
| Toxicity | LD50 orally in rabbits: 108 mg anion/kg, Dollahite, Rowe, Southwest. Vet. 27, 246 (1974) | |||||||||
| NFPA 704 |
|
POTASSIUM NITRITE price More Price(18)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 310484 | Potassium nitrite ACS reagent, ≥96.0% | 7758-09-0 | 100G | ₹21769.08 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 310484 | Potassium nitrite ACS reagent, ≥96.0% | 7758-09-0 | 100G | ₹21769.08 | 2022-06-14 | Buy |
| Sigma-Aldrich | 310484 | Potassium nitrite ACS reagent, ≥96.0% | 7758-09-0 | 500G | ₹35690.03 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 310484 | Potassium nitrite ACS reagent, ≥96.0% | 7758-09-0 | 500G | ₹35690.03 | 2022-06-14 | Buy |
| Sigma-Aldrich | 12654 | Potassium nitrite meets analytical specification of FCC, 97-100.5% | 7758-09-0 | 1KG | ₹15393.15 | 2022-06-14 | Buy |
POTASSIUM NITRITE Chemical Properties,Uses,Production
Chemical Properties
Potassium nitrite is a white to yellowish crystalline solid.
Physical properties
White or slight yellow prismatic granules; deliquesc; density 1.915 g/cm3;melts at 440°C; decomposition starts at 350°C; very soluble in water, 281 g/100mL at 0°C; much more soluble in boiling water, 413 g/100mL at 100°C;aqueous solution is alkaline; slightly soluble in cold alcohol but moderately solublel in hot alcohol; very soluble in liquid ammonia; decomposes in acids,liberating brown NO2fumes.
Uses
Potassium Nitrite is a color fixative in meats which exists as white or yellowish granules or cylindrical sticks. it is very soluble in water. see nitrite.
Preparation
Potassium nitrite may be prepared by fusion of nitrate with lead:
KNO3+ Pb →KNO2+ PbO
The product is extracted with water and allowed to crystallize. Filtration separates nitrite from insoluble lead oxide.
Potassium nitrite also may be obtained by high temperature thermal decomposition of nitrate:
2KNO3→2KNO2+ O2↑.
Production Methods
Potassium nitrite, KNO2, yellowish-white solid, soluble, for med (1) by reaction of nitric oxide plus nitrogen tetroxide and potassium carbonate or hydroxide, and then evaporating, (2) by heating potassium nitrate and lead to a high temperature and then extracting the soluble portion (lead monoxide insoluble) with H2O, and evaporating.
Definition
potassium nitrite: A white orslightly yellow deliquescent solid,KNO2, soluble in water and insolublein ethanol; r.d. 1.91; m.p. 440°C; mayexplode at 600°C. Potassium nitrite isprepared by the reduction of potassiumnitrate. It reacts with cold dilutemineral acids to give nitrousacid and is also able to behave as areducing agent (if oxidized to the nitrate)or as an oxidizing agent (if reducedto nitrogen). It is used inorganic synthesis because of its partin diazotization, and in detecting thepresence of the amino groups in organiccompounds.
General Description
A yellowish white crystalline solid. Noncombustible but accelerates the burning of all combustible material. If large quantities are involved in fire or if the combustible material is finely divided, an explosion may result. May explode under prolonged exposure to heat. Toxic oxides of nitrogen are produced in fires. Used to make other chemicals and in chemical analysis.
Air & Water Reactions
Water soluble.
Reactivity Profile
POTASSIUM NITRITE is an oxidizing agent. Mixtures with phosphorus, tin(II) chloride or other reducing agents may react explosively [Bretherick 1979 p. 108-109]. Contamination by ammonium compounds can initiate spontaneous decomposition. The resulting heat may ignite surrounding combustible material. Reacts with acids to form toxic nitrogen dioxide gas. Mixing with liquid ammonia forms diPOTASSIUM NITRITE, which is very reactive and easily explosive [Mellor 2, Supp. 3:1566 1963]. Melting together with an ammonium salt leads to a violent explosion [Von Schwartz 1918 p. 299]. A mixture with potassium cyanide may cause an explosion. When a little ammonium sulfate is added to fused POTASSIUM NITRITE, a vigorous reaction occurs attended by flame [Mellor 2:702. 1946-47].
Hazard
Fire and explosion risk when shocked or heated, or in contact with organic materials, strong oxidizing agent.
Health Hazard
Inhalation, ingestion or contact (skin, eyes) with vapors or substance may cause severe injury, burns or death. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may cause pollution.
Fire Hazard
These substances will accelerate burning when involved in a fire. Some may decompose explosively when heated or involved in a fire. May explode from heat or contamination. Some will react explosively with hydrocarbons (fuels). May ignite combustibles (wood, paper, oil, clothing, etc.). Containers may explode when heated. Runoff may create fire or explosion hazard.
Safety Profile
Poison by ingestion. Human systemic effects: tinnitus, pulse rate increase, blood pressure lowering. Experimental teratogenic and reproductive effects. Nitrites have been implicated in an increased incidence of cancer. Mutation data reported. Flammable when exposed to heat or flame. A powerful oxidzing material. Slight explosion hazard when exposed to heat. It wdl explode at 1000°F. Explosive reaction with potassium amide + heat, potassium cyanide or other cyanide salts + heat. Violent reaction or ignition with ammonium salts (e.g., ammonium sulfate), boron. Disproportionates on heating in the absence of air forming KNO3 and K2O and evolving N2. Upon decomposition it emits toxic fumes of K2O. See also NITRITES.
Potential Exposure
Potassium nitrite is used in chemical analysis, as a food additive; in fertilizers; in medications as a vasodilator and as antidote for cyanide poisoning.
Shipping
UN1488 Potassium nitrite, Hazard Class: 5.1; Labels: 5.1-Oxidizer. UN1479 Oxidizing solid, n.o.s., Hazard Class: 5.1; Labels: 5.1-Oxidizer, Technical Name Required.
Purification Methods
A saturated solution at 0o is warmed and partially evaporated under vacuum. The crystals so obtained are filtered off from the warm solution. (This procedure is designed to reduce the level of nitrate impurity and is based on the effects of temperature on solubility. The solubility of KNO3 in water is 13g/100mL at 0o, 247g/100mL at 100o; for KNO2 the corresponding figures are 280g/100mL and 413g/100mL.) Alternatively, dissolve it in H2O and precipitate by adding of EtOH.
Incompatibilities
A strong oxidizer. Reacts violently with combustible and reducing materials. Heat above 530 ? C may cause explosion. Incompatible with cyanide salts; boron, ammonium sulfate; potassium amide; and acids. Decomposes on contact with even weak acids producing toxic nitrogen oxide fumes.
POTASSIUM NITRITE Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| JSK Chemicals | +919879767970 | Gujarat, India | 3756 | 58 | Inquiry |
| Eureka Chemicals | +91-2222074176 +91-2222074206 | Maharashtra, India | 11 | 58 | Inquiry |
| BGP Group of Companies | +91-2646671200 +91-2646671200 | Gujarat, India | 3 | 58 | Inquiry |
| Shri Shanti Laboratories | +91-9799144442 +91-9571511119 | Rajasthan, India | 45 | 58 | Inquiry |
| Otto Chemie Pvt. Ltd. | +91 9820041841 | Mumbai, India | 5873 | 58 | Inquiry |
| Alfa Aesar | 1 800 209 7001 | Maharashtra, India | 6913 | 58 | Inquiry |
| Vishnupriya Chemicals Private Limited | +91-8046041737 | Telangana, India | 220 | 58 | Inquiry |
| Triveni chemicals | 08048762458 | New Delhi, India | 6093 | 58 | Inquiry |
| Central Drug House(P) Ltd. | 91-11-49404040 | New Delhi, India | 6160 | 58 | Inquiry |
| LOBA CHEMIE PVT.LTD. | 91-22-6663 6699 | Mumbai, India | 3077 | 58 | Inquiry |
| Supplier | Advantage |
|---|---|
| JSK Chemicals | 58 |
| Eureka Chemicals | 58 |
| BGP Group of Companies | 58 |
| Shri Shanti Laboratories | 58 |
| Otto Chemie Pvt. Ltd. | 58 |
| Alfa Aesar | 58 |
| Vishnupriya Chemicals Private Limited | 58 |
| Triveni chemicals | 58 |
| Central Drug House(P) Ltd. | 58 |
| LOBA CHEMIE PVT.LTD. | 58 |





