2-Nitrochlorobenzene
- CAS No.
- 88-73-3
- Chemical Name:
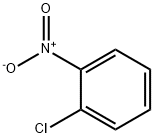
- 2-Nitrochlorobenzene
- Synonyms
- 1-CHLORO-2-NITROBENZENE;ONCB;2-CHLORONITROBENZENE;O-NITROCHLOROBENZENE;ORTHO-CHLORONITROBENZENE;2-cnb;lenvaint-F;oronitrobenzene;2-Chlornitrobenzol;o-Chloronitrobenzen
- CBNumber:
- CB3767980
- Molecular Formula:
- C6H4ClNO2
- Molecular Weight:
- 157.55
- MOL File:
- 88-73-3.mol
- MSDS File:
- SDS
- Modify Date:
- 2023/10/20 15:51:06
| Melting point | 31-33 °C (lit.) |
|---|---|
| Boiling point | 246 °C (lit.) |
| Density | 1.348 g/mL at 25 °C (lit.) |
| vapor density | 5.4 (vs air) |
| vapor pressure | 0.04 mm Hg ( 25 °C) |
| refractive index | 1.5520 (estimate) |
| Flash point | >230 °F |
| storage temp. | Store below +30°C. |
| solubility | alcohol: soluble |
| form | Fused Crystalline Mass |
| color | Yellow |
| PH | 6 (0.4g/l, H2O, 20℃) |
| explosive limit | 1.15-13.1%(V) |
| Water Solubility | 0.43 g/L (20 ºC) |
| Merck | 14,2151 |
| BRN | 509273 |
| Stability | Stable. Combustible. Incompatible with strong oxidizing agents, reducing agents, strong bases, alkali metals. |
| InChIKey | BFCFYVKQTRLZHA-UHFFFAOYSA-N |
| CAS DataBase Reference | 88-73-3(CAS DataBase Reference) |
| NIST Chemistry Reference | Benzene, 1-chloro-2-nitro-(88-73-3) |
| IARC | 2B (Vol. 65, 123) 2020 |
| EPA Substance Registry System | o-Chloronitrobenzene (88-73-3) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS06 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H301+H311 | |||||||||
| Precautionary statements | P264-P270-P280-P301+P310-P302+P352+P312-P361+P364 | |||||||||
| Hazard Codes | T | |||||||||
| Risk Statements | 22-24-52-53-52/53 | |||||||||
| Safety Statements | 36/37/39-45-60-38-28A | |||||||||
| RIDADR | UN 1578 6.1/PG 2 | |||||||||
| WGK Germany | 2 | |||||||||
| RTECS | CZ0875000 | |||||||||
| Autoignition Temperature | 500 °F | |||||||||
| Hazard Note | Toxic | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 6.1 | |||||||||
| PackingGroup | II | |||||||||
| HS Code | 29049085 | |||||||||
| NFPA 704 |
|
2-Nitrochlorobenzene price More Price(13)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | 185760 | 1-Chloro-2-nitrobenzene 99% | 88-73-3 | 250G | ₹1993.95 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 185760 | 1-Chloro-2-nitrobenzene 99% | 88-73-3 | 1KG | ₹4578.7 | 2022-06-14 | Buy |
| ALFA India | ALF-A15403-30 | 1-Chloro-2-nitrobenzene, 99% | 88-73-3 | 250g | ₹2455 | 2022-05-26 | Buy |
| ALFA India | ALF-A15403-0I | 1-Chloro-2-nitrobenzene, 99% | 88-73-3 | 5000g | ₹21258 | 2022-05-26 | Buy |
| ALFA India | ALF-A15403-0B | 1-Chloro-2-nitrobenzene, 99% | 88-73-3 | 1000g | ₹4246 | 2022-05-26 | Buy |
2-Nitrochlorobenzene Chemical Properties,Uses,Production
Chemical Properties
yellow solid with a characteristic odour
Uses
Intermediate, especially for dyes.
Production Methods
Nitration of chlorobenzene with mixed acid (30/56/14) typically gives an isomer mix in 98 % yield consisting of 34 – 36 % 2-Nitrochlorobenzene, 63 – 65 % 4- chloronitrobenzene, and only ca. 1 % 3- chloronitrobenzene. The ortho – para ratio of about 0.55 is in sharp contrast to the 1.6 obtained with nitrotoluene isomers. As with the nitration of toluene, much work has been done on isomer control so that the producer might have some flexibility towards the balance of isomer demand. Although no major change in ratio has been achieved, the situation is better for nitrochlorobenzene than nitrotoluenes, because the favored isomer is in much greater demand. In further contrast to the nitration of toluene, the nitration of chlorobenzene in the presence of phosphoric acid decreases the proportion of 4-Nitrochlorobenzene, and the use of phosphoric acid in the presence of a transition-metal catalyst is said to increase the ortho – para ratio to ca. 0.8.
General Description
Yellow crystals with an aromatic odor. Sinks in water.
Air & Water Reactions
2-Nitrochlorobenzene may be sensitive to prolonged exposure to air and light. Insoluble in water.
Reactivity Profile
O-NITROCHLOROBENZENE is incompatible with strong bases and strong oxidizing agents. 2-Nitrochlorobenzene will react with ammonia.
Hazard
Toxic by inhalation and ingestion. Combustible. Questionable carcinogen.
Health Hazard
INHALATION: Headache, languour, anemia. Irritation of nose and throat, cyanosis, shallow respiration, convulsions, and coma. EYES: Irritation. SKIN: Irritation. INGESTION: Forms methemoglobin giving rise to cyanosis and blood changes.
Safety Profile
Suspected carcinogen with experimental carcinogenic and neoplastigenic data. Poison by ingestion, skin contact, and probably inhalation. Combustible when exposed to heat or flame. To fight fire, use water, foam. Potentially explosive reaction with ammonia at 160℃/30 bar. When heated to decomposition it emits toxic fumes of Cl-, NOx, and phosgene. See also other chloronitrobenzene entries and NITRO COMPOUNDS of AROMATIC HYDROCARBONS.
Purification Methods
Crystallise it from EtOH, MeOH or pentane (charcoal). [Beilstein 5 IV 721.]
2-Nitrochlorobenzene Preparation Products And Raw materials
Raw materials
1of3
chevron_rightPreparation Products
1of7
chevron_right88-73-3(2-Nitrochlorobenzene)Related Search:
1of4
chevron_right




