Rivaroxaban
- CAS No.
- 366789-02-8
- Chemical Name:
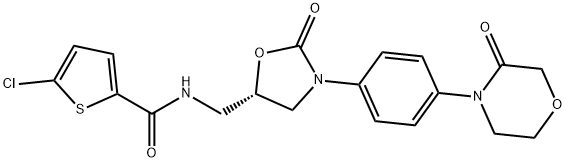
- Rivaroxaban
- Synonyms
- Xarelto;Rivaroxban;(S)-5-Chloro-N-((2-oxo-3-(4-(3-oxomorpholino)phenyl)-oxazolidin-5-yl)methyl)thiophene-2-carbox;(S)-5-chloro-N-((2-oxo-3-(4-(3-oxomorpholino)phenyl)oxazolidin-5-yl)methyl)thiophene-2-carboxamide;(S)-5-chloro-N-{[2-oxo-3-[4-(3-oxomorpholin-4-yl)phenyl]oxazolidin-5-yl]methyl} thiophene-2-carboxamide;RIVAROXABAN STAGE-IV [5-CHLORO-N-({5S)-2-OXO-3-[4-(3-OXO-4-MORPHOLINYL)PHENYL]-1,3-OXAZOLIDIN-5-YL}-METHYL)-2(AS PER INV;CS-273;Rivaroxaba;Rivarobaxan;BAY 59-7939
- CBNumber:
- CB91176772
- Molecular Formula:
- C19H18ClN3O5S
- Molecular Weight:
- 435.88
- MOL File:
- 366789-02-8.mol
- Modify Date:
- 2025/1/27 9:38:02
| Melting point | 228-229°C |
|---|---|
| Boiling point | 732.6±60.0 °C(Predicted) |
| Density | 1.460±0.06 g/cm3(Predicted) |
| storage temp. | Inert atmosphere,2-8°C |
| solubility | insoluble in H2O; insoluble in EtOH; ≥13.9 mg/mL in DMSO with gentle warming |
| form | solid |
| pka | 13.36±0.46(Predicted) |
| color | White to off-white |
| optical activity | [α]/D -34 to -44, c =0.3 in DMSO |
| InChI | InChI=1S/C19H18ClN3O5S/c20-16-6-5-15(29-16)18(25)21-9-14-10-23(19(26)28-14)13-3-1-12(2-4-13)22-7-8-27-11-17(22)24/h1-6,14H,7-11H2,(H,21,25)/t14-/m0/s1 |
| InChIKey | KGFYHTZWPPHNLQ-AWEZNQCLSA-N |
| SMILES | C1(C(NC[C@@H]2OC(=O)N(C3=CC=C(N4CCOCC4=O)C=C3)C2)=O)SC(Cl)=CC=1 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS09 |
|---|---|
| Hazard statements | H411 |
| Hazardous Substances Data | 366789-02-8(Hazardous Substances Data) |
Rivaroxaban Chemical Properties,Uses,Production
Description
Rivaroxaban is an orally active, direct inhibitor of Factor Xa (Ki = 0.4 nM), which is a crucial component of the intrinsic and extrinsic pathways of the blood coagulation cascade. It demonstrates >10,000-fold greater selectivity for Factor Xa compared to other related serine proteases (thrombin, trypsin, plasmin, FVIIa, FIXa, FXIa, urokinase, and activated protein C). In various animal arterial and venous thrombosis models, rivaroxaban is reported to inhibit thrombin formation without prolonging bleeding time and has been approved for clinical use as an anticoagulant in the prevention of stroke and the treatment of venous thromboembolisms.
Chemical Properties
White Solid.
Rivaroxaban has limited pH-independent solubility in aqueous medium (5–7 mg/L; pH 1–9), but is, for instance, slightly soluble in polyethylene glycol 400 (2,431 mg/L). Using a validated Caco-2 cell assay, the apparent permeability values of the rivaroxaban molecule at concentrations of 1–100 μM were approximately 8 × 10?6 cm/s. With a log P value (octanol/water partition) coefficient of 1.5, rivaroxaban exhibits moderate lipophilicity, reflected in its low-to-moderate affinity to peripheral tissues[1].
IUPAC: 5-chloro-N-[[(5S)-2-oxo-3-[4-(3-oxomorpholin-4-yl)phenyl]-1,3-oxazolidin-5-yl]methyl]thiophene-2-carboxamide
Uses
Rivaroxaban is a novel antithrombotic agent. It is a novel, oral, selective direct inhibitor of factor Xa developed by Bayer Healthcare. It has been approved by the EMEA and FDA for the prevention ofvenous thromboembolism in adult patients after total hip replacement or total kneereplacement surgery.
Definition
ChEBI: Rivaroxaban is a monocarboxylic acid amide obtained by formal condensation of the carboxy group of 5-chlorothiophene-2-carboxylic acid with the amino group of 4-{4-[(5S)-5-(aminomethyl)-2-oxo-1,3-oxazolidin-3-yl]phenyl}morpholin-3-one. An anticoagulant used for prophylaxis of venous thromboembolism in patients with knee or hip replacement surgery. It has a role as an anticoagulant and an EC 3.4.21.6 (coagulation factor Xa) inhibitor. It is a member of thiophenes, an organochlorine compound, an oxazolidinone, a member of morpholines, a lactam, an aromatic amide and a monocarboxylic acid amide.
Pharmacokinetics
Rivaroxaban was absorbed rapidly with maximum plasma concentrations (Cmax) being reached 2–4 h after a single dose (1.25–80 mg) and multiple doses (up to 30 mg twice daily). Rivaroxaban did not accumulate to a relevant extent after multiple dosing. Data from phase I studies in healthy subjects showed that absorption is almost complete (oral bioavailability approached 80–100 %) for the 10 mg tablet dose, irrespective of fasting or fed conditions[3]. When administered orally with food, AUC and Cmax values were similar for whole and crushed rivaroxaban 20 mg tablets, whereas a crushed tablet suspended in water, administered using a nasogastric tube and followed by a liquid meal, gave similar AUC values but an 18 % reduction in Cmax.
Side effects
Regarding safety, there was no statistical difference in the incidence of major postoperative bleeding between any of the rivaroxaban dose groups and enoxaparin although there did appear to be a dose dependency in the rivaroxaban set. In addition to bleeding and subsequent posthemorrhagic anemia, presenting as weakness, paleness, asthenia, dizziness, headache, or unexplained swelling, other common adverse events included nausea, increased GGT, and an increase in transglutaminase. Owing to its mechanism of action, there is a bleeding risk, so the drug is contraindicated in patients with clinically active bleeding. Rivaroxaban is also contraindicated in pregnant and breast-feeding women and in patients with hepatic disease associated with coagulopathy and clinically relevant bleeding risk.
Synthesis
To date, several methods have been reported for the synthesis of rivaroxaban. Most of them share the use of 5-S-hydroxymethyl or 5-S-aminomethyl oxazolidinones (2and 3 respectively) as key intermediates. Condensation of 3-morpholinone with 4-fluoronitrobenzene followed by catalytic hydrogenation provides N-(p-aminophenyl)morpholinone for subsequent reaction with (S)-2-(phthalimidomethyl)oxirane. With establishment of the aminoalcohol adduct, cyclization with 1,1′-carbonyldiimidazole generates the central oxazolidinone. Deprotection and acylation with 5-chlorothiophene-2-carbonyl chloride affords rivaroxaban.
An Improved Synthesis of Rivaroxaban
Mode of action
Rivaroxaban is a direct, specific Factor Xa inhibitor. In vitro kinetic studies showed that the inhibition of human Factor Xa by rivaroxaban was competitive [inhibition constant (K i) 0.4 ± 0.02 nM] with 10,000-fold greater selectivity than for other serine proteases—it does not inhibit related serine proteases at concentrations up to 20 μM. This compound potently inhibited prothrombinase activity [inhibitory concentration 50 % (IC50) 2.1 ± 0.4 nM], and clot-associated Factor Xa activity (IC50 75 nM). In human plasma, rivaroxaban inhibited endogenous Factor Xa activity in a concentration-dependent manner with an IC50 of 21 ± 1 nM [19]. In an ex vivo study, rivaroxaban at a single dose of 5 mg and 30 mg reduced collagen-induced endogenous thrombin potential in human plasma by approximately 80 and 90 %, respectively, and tissue factor-induced endogenous thrombin potential by approximately 40 and 65 %, respectively. In contrast to indirect Factor Xa inhibitors, rivaroxaban does not require any cofactor to exert its anticoagulant effect. In human plasma, rivaroxaban concentration-dependently inhibited thrombin generation and, thus, the amplification processes of coagulation. Thrombin generation was almost completely inhibited at therapeutically relevant concentrations (80–100 nM) of rivaroxaban. In addition, rivaroxaban increased the permeability and degradability of the whole blood clot, by decreasing thrombin generation. However, rivaroxaban does not inhibit the activity of pre-existing thrombin molecules[1-2].
References
[1] Mueck, W., et al. "Clinical Pharmacokinetic and Pharmacodynamic Profile of Rivaroxaban."Springer Open Choice 53(2014):1-16.
[2] Perzborn, E., et al. "In vitro and in vivo studies of the novel antithrombotic agent BAY 59-7939—an oral, direct Factor Xa inhibitor." Journal of Thrombosis and Haemostasis 3.3(2005):514-521.
[3] Kubitza, Dagmar , et al. "Safety, pharmacodynamics, and pharmacokinetics of BAY 59-7939—an oral, direct Factor Xa inhibitor—after multiple dosing in healthy male subjects." European Journal of Clinical Pharmacology 61.12(2005):873-80.
Rivaroxaban Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Jigs Chemical ltd | +919099003427 | Gujarat, India | 239 | 58 | Inquiry |
| ANWITA APIS | +919000311012 | Hyderabad, India | 195 | 58 | Inquiry |
| Dr. Reddy's Laboratories Ltd | +91-4049002900 +91-4049002900 | Hyderabad, India | 165 | 58 | Inquiry |
| ChemBioReas Life Sciences | +91-7989264158 +91-7989264158 | Hyderabad, India | 82 | 58 | Inquiry |
| Saptagir Laboratories PVT.LTD. | +91-9059978049 +91-9059978049 | Telangana, India | 30 | 58 | Inquiry |
| CVR Life sciences Pvt Ltd | +91-7386789224 +91-9912960099 | Hyderabad, India | 22 | 58 | Inquiry |
| Vidgas science and technologies Pvt Ltd | +91-9030019844 +91-9030019844 | Telangana, India | 76 | 58 | Inquiry |
| Metrochem API Private Limited | +91-4069069999 +91-4069069999 | Telangana, India | 78 | 58 | Inquiry |
| SVK Laboratories Pvt Ltd | +91-40-23095079 +91-9177729915 | Telangana, India | 89 | 58 | Inquiry |
| Synaptics Labs Private Limited | +91-8341963666 +91-8341963666 | Hyderabad, India | 84 | 58 | Inquiry |
Related articles
- Rivaroxaban: A Comprehensive Overview of Its Synthesis, Composition, and Applications
- Rivaroxaban is an anticoagulant medication (blood thinner) used to treat and prevent blood clots.Specifically it is used to tr....
- Oct 28,2024
- Rivaroxaban: Uses, Dose, Mechanism of action and Side effects
- Rivaroxaban is an oral antithrombotic medicine usually sold in the form of tablets and suspension (liquid).
- Jun 7,2024
- Rivaroxaban
- Rivaroxaban (Xarelto?), an oral oxazolidinone-based anticoagulant, is a potent, selective direct inhibitor of factor Xa
- Jul 26,2022
366789-02-8(Rivaroxaban)Related Search:
1of4
chevron_right




