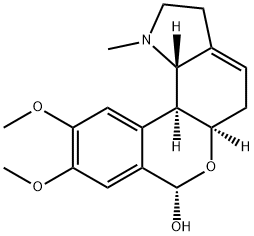
lycorenine
- CAS No.
- 477-19-0
- Chemical Name:
- lycorenine
- Synonyms
- ycorenine;lycorenine;lycorenine USP/EP/BP;9,10-Dimethoxy-1-methyllycorenan-7α-ol;(7alpha)-9,10-dimethoxy-1-methyllycorenan-7-ol;(5aR,7S,11bS,11cS)-9,10-dimethoxy-1-methyl-3,5,5a,7,11b,11c-hexahydro-2H-isochromeno[3,4-g]indol-7-ol;(5aR,7S,11bS,11cS)-1,2,3,5,5a,7,11b,11c-Octahydro-9,10-dimethoxy-1-methyl-[2]benzopyrano[3,4-g]indol-7-ol;[2]Benzopyrano[3,4-g]indol-7-ol, 1,2,3,5,5a,7,11b,11c-octahydro-9,10-dimethoxy-1-methyl-,(5aR,7S,11bS,11cS)-
- CBNumber:
- CB91377212
- Molecular Formula:
- C18H23NO4
- Molecular Weight:
- 317.38
- MOL File:
- 477-19-0.mol
- Modify Date:
- 2024/8/5 20:55:42
lycorenine Chemical Properties,Uses,Production
Description
An alkaloid isolated from Lycoris radiata Herb. the base crystallizes from Me2CO in rhombic prisms and has [α]20D + 149.3°. The alkaloid yields crystalline salts and derivatives, e.g. the aurichloride decomposing at 116°C, platinichloride, also decomposing before melting at 210°C; picrate, m.p. 162°C (dec.); monoacetyl derivative, m.p. 185 _7°C. The diacetyl compound has been prepared although with some difficulty and under forcing conditions, m.p. 17 5-6°C. The alkaloid behaves as a pseudo-base giving an oxime hydrochloride which decomposes at 258°C. On catalytic hydrogenation it yields the dihydro-derivative, m.p. 175- 7°C and prolonged hydrogenation eventually forms deoxytetrahydrolycorenine, ClsH2S03N, m.p. 165-8°C although other compounds are also formed at the same time, e.g. C1sH27 0 2N, m.p. l65-7°C and ClsH2S03N, m.p. l20-3°C. The methiodide decomposes when heated at 260°C and on treatment with silver oxide forms two methine bases, of which the (±)-form has been isolated as the methiodide, m.p. 223°C (dec.). The complete structure has been determined primarily from the mass spectrum.
Uses
Lycorenine is an antibacterial agent extracted from the bulb of Lycoris radiata.
References
Kondo, Tomimura, Ishiwatari., J. Pharrn. Soc., Japan, 52, 51 (1932) Spectra: Inuka et aI., Tetrahedron Lett., 4745 (1966) Stereochemistry: Mizukami., Tetrahedron, 11,89 (1960)
lycorenine Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Pharmaffiliates Analytics and Synthetics P. Ltd | +91-172-5066494 | Haryana, India | 6773 | 58 | Inquiry |
| Pharma Affiliates | 172-5066494 | Haryana, India | 6761 | 58 | Inquiry |
| Shaanxi Dideu Medichem Co. Ltd | 18192627656 | China | 2788 | 58 | Inquiry |
| Chengdu GLP biotechnology Co Ltd | 028-87075086 13350802083 | CHINA | 1824 | 58 | Inquiry |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | China | 49391 | 58 | Inquiry |
| Hefei TNJ Chemical Industry Co.,Ltd. | 0551-65418671 | China | 34571 | 58 | Inquiry |
| Dideu Industries Group Limited | +86-29-89586680 +86-15129568250 | China | 24407 | 58 | Inquiry |
| Hangzhou MolCore BioPharmatech Co.,Ltd. | +86-057181025280; +8617767106207 | China | 49739 | 58 | Inquiry |
| Hebei Guanlang Biotechnology Co., Ltd. | +8619930503259 | China | 18433 | 58 | Inquiry |
| TargetMol Chemicals Inc. | +1-781-999-5354 | United States | 19973 | 58 | Inquiry |
477-19-0(lycorenine)Related Search:
1of4
chevron_right




