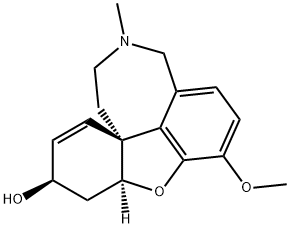
Galanthamine
- CAS No.
- 357-70-0
- Chemical Name:
- Galanthamine
- Synonyms
- GALANTAMINE;Galantamin;Razadyne;Galantamina;Galanthamine-d3 HCl;(4aS,8aS)-4a,5,9,10,11,12-Hexahydro-3-methoxy-11-methyl-6H-benzofuro[3a,3,2-ef][2]benzazepin-6β-ol;Jilkon;ReMinyl;R-113675;Nevalina
- CBNumber:
- CB8345888
- Molecular Formula:
- C17H21NO3
- Molecular Weight:
- 287.35
- MOL File:
- 357-70-0.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/7/26 17:49:17
| Melting point | 119-1210C |
|---|---|
| alpha | D20 -118.8° (c = 1.378 in ethanol) |
| Boiling point | 429.65°C (rough estimate) |
| Density | 1.0662 (rough estimate) |
| refractive index | 1.5022 (estimate) |
| storage temp. | Sealed in dry,Room Temperature |
| solubility | ≥14.37 mg/mL in DMSO; ≥14.43 mg/mL in H2O with gentle warming; ≥45 mg/mL in EtOH with gentle warming |
| form | Powder |
| pka | pKa 8.32 (Uncertain) |
| color | White to off-white |
| CAS DataBase Reference | 357-70-0(CAS DataBase Reference) |
| NIST Chemistry Reference | Galantamin(357-70-0) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS09,GHS06 |
|---|---|
| Signal word | Danger |
| Hazard statements | H319-H373-H410-H317-H315-H301+H331 |
| Precautionary statements | P261-P272-P280-P302+P352-P333+P313-P321-P363-P501-P260-P314-P501-P264-P280-P302+P352-P321-P332+P313-P362-P264-P280-P305+P351+P338-P337+P313P-P273-P391-P501 |
| Toxicity | LD50 intraperitoneal in mouse: 10mg/kg |
Galanthamine Chemical Properties,Uses,Production
Description
Galantamine is an Amaryllidaceae alkaloid which is first obtained from the plant of
snowdrop (Galanthus nivalis), and nowadays, it’s extracted from the plants of
Narcissus and Galanthus species or obtained from chemosynthesis. In many
areas in Europe such as Bulgaria, eastern Turkey, and the Caucasus, the plants of
Galanthus are native species. But its earliest pharmaceutical applications are seldom known. Plaitakis and Duvoisin hypothesized that “moly” in ancient Homer’s
epic might be snowdrops. In Homer’s epic Odyssey, “moly” was used as an antidote
by Odysseus against Circe’s poisonous drugs. To be used as an antidote may be the
plants of Galanthus’ oldest medicinal records. But there is not much evidence for
that. There is little evidence of the traditional application of the plants of
Galanthus, and it is certain that until the Second World War, the plants of Galanthus
and other genera of Amaryllidaceae were not frequently used in European drugs
Italian scholar Iannello studied Pancratium illyricum L., an Amaryllidaceae species endemic to Sardinia, and isolated a new galantamine-type alkaloid in its leaves.
This new galantamine-type compound exhibited a pronounced in?vitro AChE inhibitory activity (IC50? =? 3.5±1.1? μM) in comparison with the reference standard
galantamine hydrobromide (IC50?=?1.5±0.2?μM)
Chemical Properties
Off-White Solid
Physical properties
Hydrobromide of galantamine can be used as drug although galantamine can’t be
used as drugs
Appearance: an almost white powder. Solubility: soluble in water; slightly soluble in alcohol; and insoluble in acetone, trichloromethane, and diethyl ether.
Melting point: 269–270?°C. Specific optical rotation: ?90 to ?100°.
Occurrence
Galanthamine is a bulb plant found throughout the world.
History
In the early 1950s, Soviet Union’s scientists started modern medicine research of
galantamine. In 1951, the Soviet Union’s pharmacologist Mashkovsky and
Kruglikova-Lvova firstly proved its AChE-inhibiting properties, which was published in the paper. In 1957, the Bulgarian pharmacologist Paskov et?al. found that
the plants of Galanthus were the richest sources of galantamine, which opened a
way for its commercial development by the company Sopharma in Bulgaria.
Galantamine hydrobromide was launched into market under the trade name Nivalin.
Initially, Nivalin was used in anesthesia to antagonize the effects of non-depolarizing
muscle relaxants, and since then, it was rapidly introduced in other areas of
medicine
Galantamine hydrobromide was launched into market for the indication of mild
to moderate Alzheimer’s disease firstly in 1995, developed by Sanochemia
Pharmaceuticals. In the United States, galantamine hydrobromide was developed
by Janssen Pharmaceuticals, a subsidiary of Johnson & Johnson, and was listed
under the trade name Razadyne in 2001. So far, galantamine hydrobromide has
been marketed in more than 20 countries and regions, such as the United States,
Europe, and Japan.
Uses
A selective acetylcholinesterase inhibitor. Useful for the treatment of Alzheimer's disease.
Indications
Its formulation includes capsule, tablet, and oral solution. It is used in the symptomatic treatment of mild to moderately severe dementia in Alzheimer’s disease.
Definition
ChEBI: A natural product found in Crinum asiaticum var. sinicum.
General Description
Galantamine, 4a,5,9,10,11,12-hexahydro-3-methoxy-11-methyl-6H-benzofuro-[3a,3,2,ef][2]-benzazepin-6-ol (Nivalin, Reminyl), is an alkaloid extractedfrom the tuberous plant Leucojum aestivum (L.) belongingto the Amaryllidaceae family and from the bulbs of thedaffodil, Narcissus pseudonarcissus. It is a reversiblecholinesterase inhibitor that appears to have no effect on butyrylcholinesterase.In addition, it acts at allosteric nicotinicsites, further enhancing its cholinergic activity. Galantamineundergoes slow and minor biotransformation with approximately5% to 6% undergoing demethylation. It is primarilyexcreted in the urine.
Clinical Use
Before the 1990s, galantamine was mainly used to treat poliomyelitis sequelae,
muscle atrophy, postoperative intestinal muscle paralysis, urinary retention, and
myasthenia gravis. In the 1990s, it was found that galantamine had improved memory impairment in mice, suggesting that it might be effective for central cholinergic
disorders in Alzheimer’s disease
In a 3–6?months’ well-designed clinical trial, recipients of galantamine achieved
significant improvements in cognitive symptoms compared to placebo recipients.
Galantamine also improved activities of daily living in these patients and significantly reduced the requirement for caregiver assistance with activities of daily living. Clinical development of new indications of galanthamine is currently
underway, such as smoking cessation and improving cognitive impairment in
schizophrenia and mania.
Galanthamine Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Global Pharma | +91-9819994077 +91-9819994077 | Maharashtra, India | 34 | 58 | Inquiry |
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6351 | 58 | Inquiry |
| Rhyme Organics And Chemicals Limited | 08048372046Ext 929 | Hyderabad, India | 102 | 58 | Inquiry |
| Mahir Technologies Inc. | 09930610606 | Mumbai, India | 38 | 58 | Inquiry |
| Dabla Biotech | 09905481865 | Delhi, India | 1 | 58 | Inquiry |
| Pharmaffiliates Analytics and Synthetics P. Ltd | +91-172-5066494 | Haryana, India | 6773 | 58 | Inquiry |
| Triveni chemicals | 08048762458 | New Delhi, India | 6093 | 58 | Inquiry |
| Pharma Affiliates | 172-5066494 | Haryana, India | 6761 | 58 | Inquiry |
| Refsyn Biosciences Pvt., Ltd. | 91-413-2200273 | Tamil Nadu, India | 226 | 58 | Inquiry |
| Hebei Guanlang Biotechnology Co,.LTD | +86-19930503253; +8619930503252 | China | 5832 | 58 | Inquiry |





