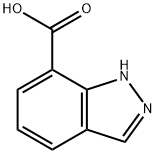
1H-indazole-7-carboxylic acid
- CAS No.
- 677304-69-7
- Chemical Name:
- 1H-indazole-7-carboxylic acid
- Synonyms
- 7-Carboxy-1H-indazole;CgH6N2O2;Niraparib Intermediate4;7-INDAZOLE CARBOXYLIC ACID;7-INDAZOLE CARBOXYLLIC ACID;1H-Indazole-7-carboxylic aid;1H-Indazol-7-carboxylic acid;1H-INDAZOLE-7-CARBOXYLIC ACID;7-(1H)Indazole carboxylic acid;677304-69-7
- CBNumber:
- CB9197275
- Molecular Formula:
- C8H6N2O2
- Molecular Weight:
- 162.15
- MOL File:
- 677304-69-7.mol
- Modify Date:
- 2023/12/25 17:47:29
| Melting point | ca 240℃ |
|---|---|
| Boiling point | 443.7±18.0 °C(Predicted) |
| Density | 1.506±0.06 g/cm3(Predicted) |
| storage temp. | Sealed in dry,Room Temperature |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| form | Crystalline Powder |
| pka | 3.71±0.10(Predicted) |
| color | White to yellow |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H315-H319-H335 | |||||||||
| Precautionary statements | P261-P280a-P304+P340-P305+P351+P338-P405-P501a | |||||||||
| HS Code | 29339980 | |||||||||
| NFPA 704 |
|
1H-indazole-7-carboxylic acid Chemical Properties,Uses,Production
Uses
1H-indazole-7-carboxylic Acid is an inhibitor of nitric oxide synthases.
Preparation
Synthesis of 1H-indazole-7-carboxylic acid, H2L4: To 60 mL anhydrous toluene under a nitrogen atmosphere was added methyl-2-amino-3-methylbenzoate (1.8 g, 11 mmol) and KOAc (560 mg, 5.7 mmol), and the mixture heated to reflux, at which time acetic anhydride (3.2 mL, 34 mmol) was added and the mixture stirred at reflux for 10 min. Isoamyl nitrite (2.3 mL, 18 mmol) was added over 30 min and the mixture refluxed overnight. On cooling, the mixture was filtered and evaporated to dryness to give 1.6 g of a pale brown solid, which analysed for methyl 1H-indazole-7-carboxylate. This material was taken up in 40 mL THF, added to a solution of LiOH (5 g, 210 mmol) in 40 mL water, and heated at reflux for 48 h. On cooling, the mixture was concentrated on a rotary evaporator and the resulting aqueous phase filtered and adjusted to pH 4 with dilute HCl, to precipitate the product, which was filtered, washed with water and dried under vacuum. Yield 810 mg, 45%; mp 218–222 °C (decomp);
δH (500 MHz, d6-DMSO): 7.23 (t, 1H, J = 7.5 Hz, H4 ), 7.97 (dd, 1H, J = 7.5, 1.0 Hz, H3 ), 8.06 (dd, 1H, J = 7.8, 0.8 Hz, H5 ), 8.21 (s, 1H, H2 ), 13.1 (br s, 2H, H1 & H6 ); δc (125 MHz, d6-DMSO): 113.7, 120.1, 124.6, 126.5, 129.0, 134.3, 138.0, 167.0; HRMS-ESI m/z: found: 163.0503; C8H7N2O2 requires: 163.0502 [M + H+ ]; ˉνmax/cm?1 (KBr): 3316 s br, 1700 s, 1619 m, 1592 m, 1509 m, 1285 s, 1201 m, 1145 m, 1078 m, 1056 w, 943 m, 858 s, 745 s, 638 m, 601 m.
1H-indazole-7-carboxylic acid Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| GLR Innovations | +91 9891111994 | New Delhi, India | 4542 | 58 | Inquiry |
| VGS SYNTHESIS PRIVATE LIMITED | +91-9866975588 +91-9866975588 | Hyderabad, India | 1818 | 58 | Inquiry |
| AATAMI Molecular Research Laboratories Pvt Ltd (AMRL) | +91-8985184986 +91-8148739767 | Telangana, India | 985 | 58 | Inquiry |
| Santo Righello Pvt. Ltd | 91-9885571757 | Hyderabad, India | 919 | 58 | Inquiry |
| Reax Chemicals | 08048372701Ext 416 | Hyderabad, India | 4223 | 58 | Inquiry |
| A.J Chemicals | 91-9810153283 | New Delhi, India | 6124 | 58 | Inquiry |
| Taizhou Blazer Biomedical Technology Co., Ltd. | 0523-86112008 13915404123 | China | 88 | 58 | Inquiry |
| Hebei Mojin Biotechnology Co., Ltd | +86 13288715578 +8613288715578 | China | 12459 | 58 | Inquiry |
| Hebei Yanxi Chemical Co., Ltd. | +86-17531190177; +8617531190177 | China | 6011 | 58 | Inquiry |
| Shaanxi Haibo Biotechnology Co., Ltd | +undefined18602966907 | China | 1000 | 58 | Inquiry |
677304-69-7(1H-indazole-7-carboxylic acid)Related Search:
1of4
chevron_right




