Valrubicin
- CAS No.
- 56124-62-0
- Chemical Name:
- Valrubicin
- Synonyms
- ad32;CS-703;Valstar;Valtaxin;VALRUBICIN;nsc-246131;Aids004409;Aids-004409;antibioticad32;Valrubicin(R&D)
- CBNumber:
- CB9425095
- Molecular Formula:
- C34H36F3NO13
- Molecular Weight:
- 723.64
- MOL File:
- 56124-62-0.mol
- Modify Date:
- 2024/11/19 20:33:22
| Melting point | 116-117 °C |
|---|---|
| Boiling point | 135-136 C |
| Density | 1.3473 (estimate) |
| storage temp. | 2-8°C |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| form | Solid |
| pka | 7.34±0.60(Predicted) |
| color | Red |
| Water Solubility | insoluble |
| InChIKey | ZOCKGBMQLCSHFP-XGMQQLFPNA-N |
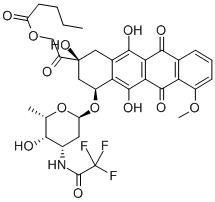
| SMILES | C12=C(O)C3=C(C(=O)C4C=CC=C(OC)C=4C3=O)C(O)=C1C[C@@](O)(C(=O)COC(=O)CCCC)C[C@@H]2O[C@@H]1O[C@H]([C@@H](O)[C@@H](NC(=O)C(F)(F)F)C1)C |&1:21,34,36,38,39,41,r| |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS08,GHS06 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H351-H340-H312-H332-H360-H301 | |||||||||
| Precautionary statements | P201-P202-P281-P308+P313-P405-P501-P264-P270-P301+P310-P321-P330-P405-P501-P261-P271-P304+P340-P312-P280-P302+P352-P312-P322-P363-P501 | |||||||||
| Hazard Codes | Xi | |||||||||
| Risk Statements | 36/37/38 | |||||||||
| Safety Statements | 26-37/39 | |||||||||
| HS Code | 2941906000 | |||||||||
| Hazardous Substances Data | 56124-62-0(Hazardous Substances Data) | |||||||||
| Toxicity | dnd-hmn:lym 3 mg/L CJBIAE 58,720,80 | |||||||||
| NFPA 704 |
|
Valrubicin Chemical Properties,Uses,Production
Description
Valrubicin was launched as a new 'chemotherapeutic agent for the treatment of bladder cancer, particularly in patients with BCG-refractory carcinoma in situ (CIS) of the bladder for whom immediate cystectomy is unacceptable. It belongs to the class of anthracyclines, the widest used in human cancers, and is a N-trifluoroacetyl 14-valerate derivative of doxorubicin.Valrubicin can be obtained in 3 steps from daunomycin by N-trifluoroacetylation of the sugar moiety then iodination of the 2-acetyl group and introduction of a valerate residue. A proposed mechanism involved in the cytotoxicity of Valrubicin coud be the blockade of SV40 large T antigen helicase; this cellular enzyme is involved in the formation of a ternary complex with DNA to maintain the topographic structure of DNA during transcription. In patients with CIS of bladder refractory to front line and second line therapies, intravesical instillation of Valrubicin resulted in a complete response with a significant rate and allowed a delay in cystectomy. Systemic absorption was minimal and accordingly produced a lower incidence of cardiotoxicity compared with doxorubicin.
Chemical Properties
Red Solid
Uses
Chemotherapy drug used to treat cancer of the bladder.
General Description
Valrubicin is available in 200-mg vials for intravesicular administrationin the treatment of bladder cancer (orphan drugstatus). The increased lipophilicity associated with the valericacid ester and trifluoro acetate functionalities increasestissue penetration and remains intact because, in large measure,of the lack of exposure to hydrolyzing enzymes causedby direct delivery into the bladder followed by voiding ofthe instilled solution. This local action also minimizes cardiotoxicityand other adverse effects seen with other anthracyclines.The major adverse effects that are seen are bladderirritation and reddening of the urine.
Mechanism of action
Valrubicin is an anthracycline that affects a variety of interrelated biological functions, most of which involve nucleic acid metabolism. In cells, it inhibits the incorporation of nucleosides into nucleic acids, causes chromosomal damage, and arrests the cell cycle in G2. Although valrubicin does not bind strongly to DNA, valrubicin metabolites interfere with the normal DNA breaking-resealing action of DNA topoisomerase II.
Clinical Use
Valrubicin currently has orphan drug status in the treatment of bacille Calmette-Guérin (BCG)–refractory bladder cancer (the total patient population is ~1,000 individuals) and is used with patients for whom surgical intervention would result in high morbidity or death.
Side effects
The most commonly reported adverse reactions are abdominal pain, urinary tract infection, hematuria, and dysuria. Systemic exposure to the drug and its metabolites would, of course, be greater in patients whose bladder wall integrity has been compromised by disease, and these patients should not receive valrubicin.
Safety Profile
Poison by intraperitoneal route.Human mutation data reported. When heated todecomposition it emits toxic fumes of Fí and NOx.
Metabolism
It is administered directly into the bladder through a catheter (intravesically). The lipophilic drug is water insoluble, but it dissolves in an aqueous vehicle that includes polyoxyethylene glycol and ethanol. The patient retains the drug in the bladder for 2 hours, then voids the solution in the normal fashion. Valrubicin is active as administered, and despite the fact that hydrolysis of the ester and trifluoroacetamide can be envisioned, it is excreted essentially unchanged. Less than 1% of an administered dose is absorbed systemically, so there is essentially no exposure to metabolizing enzymes. The reduced C13-alcoholic metabolite does not form to any appreciable extent during the 2-hour treatment period. Therapy is considered to be almost exclusively local, and there is little risk for cardiac toxicity, bone marrow suppression, drug–drug interactions, or other side effects.
Valrubicin Preparation Products And Raw materials
Raw materials
1of2
chevron_rightPreparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| OCEAN TRADING CORPORATION | +91(22) 24921669 | New Delhi, India | 6202 | 58 | Inquiry |
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6257 | 58 | Inquiry |
| A.J Chemicals | 91-9810153283 | New Delhi, India | 6100 | 58 | Inquiry |
| Pharmaffiliates Analytics and Synthetics P. Ltd | +91-172-5066494 | Haryana, India | 6739 | 58 | Inquiry |
| Pharma Affiliates | 172-5066494 | Haryana, India | 6754 | 58 | Inquiry |
| career henan chemical co | +86-0371-86658258 +8613203830695 | China | 29859 | 58 | Inquiry |
| Xiamen AmoyChem Co., Ltd | +86-86-5926051114 +8615060885618 | China | 6383 | 58 | Inquiry |
| BOC Sciences | +1-631-485-4226 | United States | 19552 | 58 | Inquiry |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | China | 49732 | 58 | Inquiry |
| SHANGHAI T&W PHARMACEUTICAL CO., LTD. | +86-021-61551413 +8618813727289 | China | 5738 | 58 | Inquiry |
56124-62-0(Valrubicin)Related Search:
1of4
chevron_right




