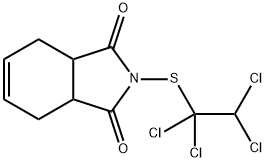
CAPTAFOL
- CAS No.
- 2425-06-1
- Chemical Name:
- CAPTAFOL
- Synonyms
- Sulfonimide;cs5623;Folcid;haipen;FOLTAF;Cs 5623;Difosan;kenofol;Sanspor;Captatol
- CBNumber:
- CB9773422
- Molecular Formula:
- C10H9Cl4NO2S
- Molecular Weight:
- 349.06
- MOL File:
- 2425-06-1.mol
- Modify Date:
- 2024/3/14 15:18:32
| Melting point | 160-161° |
|---|---|
| Boiling point | 365.7±52.0 °C(Predicted) |
| Density | 1.4682 (rough estimate) |
| vapor pressure | 1.1 x 10-6 Pa (20 °C) |
| refractive index | 1.6000 (estimate) |
| Flash point | >100 °C |
| storage temp. | 0-6°C |
| solubility | Chloroform (Sparingly), Dichloromethane (Very Slightly), Methanol (Slightly) |
| form | Solid |
| pka | -2.67±0.20(Predicted) |
| Water Solubility | 1.4 mg l-1 (20 °C) |
| color | White to Off-White |
| Merck | 13,1777 |
| IARC | 2A (Vol. 53) 1991 |
| EPA Substance Registry System | Captafol (2425-06-1) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS08,GHS09 |
|---|---|
| Signal word | Danger |
| Hazard statements | H317-H350-H410 |
| Precautionary statements | P261-P272-P280-P302+P352-P333+P313-P321-P363-P501-P273-P391-P501 |
| Hazard Codes | T,N |
| Risk Statements | 45-43-50/53 |
| Safety Statements | 53-45-60-61 |
| RIDADR | UN3077 9/PG 3 |
| OEB | D |
| OEL | TWA: 0.1 mg/m3 [skin] |
| RTECS | GW4900000 |
| HS Code | 29305000 |
| Toxicity | LD50 in rats, rabbits (mg/kg): 2500-6200 orally; 15400 dermally (Ben-Dyke) |
CAPTAFOL Chemical Properties,Uses,Production
Chemical Properties
Captafol is a white crystalline solid.
Uses
Captafol is used to control a wide range of fungal diseases on many crops.
Definition
ChEBI: A dicarboximide that captan in which the trichloromethyl group is replaced by a 1,1,2,2-tetrachloroethyl group. A broad-spectrum fungicide used to control diseases in fruit and potatoes, it is no longer approved for use in the European Community.
General Description
White crystalline solid with a slight, but pungent odor. Mp: 162°C. Practically insoluble in water. Only slightly soluble in organic solvents. Technical CAPTAFOL is a wettable light tan powder that is used as a fungicide. Inhaled dust irritates the respiratory tract. Irritates skin and damages eyes. Acute oral toxicity in humans is low. Not persistent in the environment (decomposes with a half-life of 11 days in the soil). Highly toxic to fish and other aquatic organisms.
Reactivity Profile
CAPTAFOL is non-flammable but, on heating, may decompose to generate toxic fumes, such as sulfur oxides, hydrogen sulfide, hydrochloric acid, and phosgene. Stable at room temperature when dry but readily hydrolysed, especially in an alkaline environment. CAPTAFOL and mixtures containing high concentrations of CAPTAFOL may react violently with alkali. Incompatible with acids, acid chlorides, acid anhydrides, and strong oxidizing agents. Sulfhydryl compounds such as glutathione and cysteine cause a rapid chemical decomposition.
Hazard
Absorbed by skin. Probable carcinogen.
Agricultural Uses
Fungicide: Captafol is a General Use Pesticide and used for the control of practically all forms of fungal diseases except powdery mildew. It is also used as a seed protectorant on cotton, rice and peanut crops. Not registered for use in the U.S. or in EU countries. There are 20 global suppliers.
Trade name
CAPTATOL®; CAPTOFOL®; CRISFOLATAN®; DIFOLATAN®[C]; DIFOCAP®[C]; DIFOSAN®; FOLCID®; HAIPEN®; KENOFOL®; MERPAFOL®; ORTHO® 5865[C]; PILLARTAN®; SANSEAL®; SANSPOR®; SANTAR-SM®; SULFONIMIDE®; SULPHEIMIDE®
Contact allergens
Captafol is a pesticide, belonging to thiophthalimide group. Occupational contact dermatitis was reported in an agricultural worker who had multiple sensitizations
Safety Profile
Confirmed carcinogen with experimental carcinogenic data. Poison by intraperitoneal route. Moderately toxic by ingestion. An experimental teratogen. Other experimental reproductive effects. Mutation data reported. A fungicide. When heated to decomposition it emits very toxic fumes of Cl-, NO,, and SOx
Potential Exposure
Captafol is a thiophthalimide fungicide. Those engaged in the manufacture, formulation, and application of this fungicide. Captafol is not currently registered for use on field crops or stored produce in the United States.
Carcinogenicity
Captafol is reasonably anticipated to be a human carcinogen based on sufficient evidence of carcinogenicity from studies in experimental animals and supporting data on mechanisms of carcinogenesis.
Environmental Fate
The primary toxicity following captafol exposure probably occurs through a hypersensitivity mechanism. Most experiments suggest captafol to be DNA active.
Metabolic pathway
Captafol contains an unstable tetrachloroethylthio (sulfenyl) moiety that has been shown to undergo rapid hydrolytic and metabolic degradation to tetrahydrophthalimide (2). By analogy with captan, presumably the tetrachloroethylthio moiety can be transferred to the sulfur atoms of thiols such as cysteine and glutathone. Thus in the presence of thiols such as glutathione, captafol is probably cleaved at the N-S bond to form thiophosgene (3) and other gaseous products such as hydrogen sulfide, hydrogen chloride and carbonyl sulfide. Thiophosgene is rapidly hydrolysed by water. The tetrachloroethylthio group and thiophosgene are believed to be intermediates in the formation of thiazolidine-2- thione-6carboxylic acid (4) which is an addition product with cysteine. A thiazolidine derivative of glutathione is also formed (5). Biotransformation of captafol in mammals generates primarily thiophosgene (3) and tetrahydrophthalimide (2). Tetrahydrophthalimide (2) and various of its derivatives are excreted in the urine. There were no reports of 2-thiazolidinethione-4-carboxylic acid (4) in the urine.
Shipping
UN2811 Toxic solids, organic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required. UN 2773 Phthalimide derivative pesticides, solid, toxic, Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required.
Incompatibilities
Reacts violently with bases, causing fire and explosion hazard. Not compatible with strong acids or acid vapor, oxidizers. Strong alkaline conditions contribute to instability. Attacks some metals.
Waste Disposal
Hydrolysis.
CAPTAFOL Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6351 | 58 | Inquiry |
| Chongqing Chemdad Co., Ltd | +86-023-6139-8061 +86-86-13650506873 | China | 39916 | 58 | Inquiry |
| Hebei Guanlang Biotechnology Co., Ltd. | +8619930503259 | China | 18424 | 58 | Inquiry |
| SUZHOU SENFEIDA CHEMICAL CO.,LTD | +86-0512-83500002 +8615195660023 | China | 23053 | 58 | Inquiry |
| LGC Standards | +44 (0)20 8943 8480 | United Kingdom | 6495 | 0 | Inquiry |
| Shaanxi Didu New Materials Co. Ltd | +86-89586680 +86-13289823923 | China | 8673 | 58 | Inquiry |
| Nanjing Sunlida Biological Technology Co., Ltd. | 025-57798810 | China | 3239 | 55 | Inquiry |
| Nantong Zhonghe Chemical New Materials Co., Ltd | 13003551299 13003551299 | China | 7567 | 58 | Inquiry |
| ShangHai Anpel Co, Ltd. | 18501792038; 18501792038 | China | 9614 | 58 | Inquiry |
| United States Biological | 800.520.3011 or 781.639.5092 | United States | 6256 | 80 | Inquiry |
Related articles
- Use of Captafol
- Captafol is colorless to pale yellow in color with a distinct odor. It has a molecular weight of 349.1, water solubility of 1.....
- Dec 6,2021





