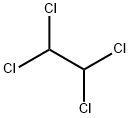
1,1,2,2-Tetrachloroethane
- CAS No.
- 79-34-5
- Chemical Name:
- 1,1,2,2-Tetrachloroethane
- Synonyms
- Cellon;1,1,2,2-tce;S-TETRACHLOROETHANE;1,1,2,2-Tetrachlorethan;ACETYLENE TETRACHLORIDE;R-130;Westrol;Westron;(CHCl2)2;Acetosol
- CBNumber:
- CB9384693
- Molecular Formula:
- C2H2Cl4
- Molecular Weight:
- 167.85
- MOL File:
- 79-34-5.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/3/19 16:34:00
| Melting point | -43 °C |
|---|---|
| Boiling point | 147 °C(lit.) |
| Density | 1.586 g/mL at 25 °C(lit.) |
| vapor density | 5.8 (vs air) |
| vapor pressure | 8 mm Hg ( 20 °C) |
| refractive index |
n |
| Flash point | 142-146°C |
| storage temp. | Store below +30°C. |
| solubility | 2830g/l |
| form | Liquid |
| color | slightly green-yellow |
| Water Solubility | 0.3 g/100 mL (25 ºC) |
| Merck | 14,9189 |
| BRN | 969206 |
| Henry's Law Constant | 6.22 at 30 °C (headspace-GC, Sanz et al., 1997) |
| Exposure limits | Potential occupational carcinogen. NIOSH REL: TWA 1 ppm (7 mg/m3), IDLH 100 ppm; OSHA PEL: TWA 5 ppm (35 mg/m3); ACGIH TLV: TWA 1 ppm (adopted). |
| Dielectric constant | 8.4199999999999999 |
| Stability | Stable. Incompatible with strong oxidizing agents, strong bases. Reacts violently with sodium, potassium, nitrates, 2,4-dinitrophenyl disulphide. |
| CAS DataBase Reference | 79-34-5(CAS DataBase Reference) |
| NIST Chemistry Reference | Ethane, 1,1,2,2-tetrachloro-(79-34-5) |
| IARC | 2B (Vol. 20, Sup 7, 71, 106) 2014 |
| EPA Substance Registry System | 1,1,2,2-Tetrachloroethane (79-34-5) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS06,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H310+H330-H411 | |||||||||
| Precautionary statements | P262-P273-P280-P302+P352+P310-P304+P340+P310 | |||||||||
| Hazard Codes | T+,N,T,F | |||||||||
| Risk Statements | 26/27-51/53-59-39/23/24/25-23/24/25-11 | |||||||||
| Safety Statements | 38-45-61-36/37-16-7 | |||||||||
| RIDADR | UN 1702 6.1/PG 2 | |||||||||
| OEB | B | |||||||||
| OEL | TWA: 1 ppm (7 mg/m3) [skin] (Chloroethanes) | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | KI8575000 | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 2903 19 00 | |||||||||
| HazardClass | 6.1 | |||||||||
| PackingGroup | II | |||||||||
| Toxicity | LD50 orally in rats: 0.20 ml/kg (Smyth) | |||||||||
| IDLA | 100 ppm | |||||||||
| NFPA 704 |
|
1,1,2,2-Tetrachloroethane price More Price(17)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | 8.43948 | 1,1,2,2-Tetrachloroethane for synthesis | 79-34-5 | 250ML | ₹3100 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 185434 | 1,1,2,2-Tetrachloroethane reagent grade, ≥98% | 79-34-5 | 1L | ₹10138.55 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 185434 | 1,1,2,2-Tetrachloroethane reagent grade, ≥98% | 79-34-5 | 500ML | ₹11520.6 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 46259 | 1,1,2,2-Tetrachloroethane analytical standard | 79-34-5 | 5ML | ₹6783.65 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 185434 | 1,1,2,2-Tetrachloroethane reagent grade, ≥98% | 79-34-5 | 2.5L | ₹27662.1 | 2022-06-14 | Buy |
1,1,2,2-Tetrachloroethane Chemical Properties,Uses,Production
Description
1,1,2,2-Tetrachloroethane (tetrachloroethane) is a volatile, synthetic, colorless to pale-yellow dense liquid with a pungent, chloroform-like odor that is soluble in water and most organic solvents. There are no known natural sources of tetrachloroethane. Tetrachloroethane is a chemical intermediate in the production of a variety of other common chemicals. In the past, the major use for tetrachloroethane was in the production of trichloroethylene, tetrachloroethylene, and 1,2-dichloroethylene. With the development of new processes for manufacturing chlorinated ethylenes and the availability of less-toxic solvents, the production of tetrachloroethane as a commercial endproduct in the United States and Canada has steadily declined since the late 1960s and the production ceased by the early 1990s. Although at one time it was used as an insecticide, fumigant, and weed killer, it presently is not registered for any of these purposes. Its registration as an insect repellent was canceled by the Environmental Protection Agency (EPA) in the late 1970s.
Chemical Properties
colourless to light yellow liquid with a chloroform-like
Physical properties
Colorless to pale yellow liquid with a sweet, chloroform-like odor. A detection odor threshold concentration of 50 mg/m3 (7.3 ppmv) was experimentally determined by Dravnieks (1974).
Uses
1,1,2,2-Tetrachloroethane, once used as a solvent for cleaning and extraction processes, is still used to some extent as a chemical intermediate. Present usage is quite limited because less toxic solvents are available.
Definition
ChEBI: A member of the class of chloroethanes that is ethane substituted by chloro groups at positions 1, 1, 2 and 2.
General Description
Colorless to pale yellow liquid with a sweet odor. Sinks in water.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
1,1,2,2-Tetrachloroethane may be incompatible with strong oxidizing and reducing agents. Also may be incompatible with many amines, nitrides, azo/diazo compounds, alkali metals, and epoxides. Decomposed by heat and UV light, forming phosgene and HCl; reacts violently with finely dispersed metals [Handling Chemicals Safely 1980. p. 886].
Hazard
Toxic by ingestion, inhalation, skin absorption. Questionable carcinogen.
Health Hazard
Compound is a powerful narcotic and liver poison; may also cause changes in blood composition and neurological disturbances. Repeated exposure by inhalation can be fatal. Ingestion causes vomiting, diarrhea, severe mucosal injury, liver necrosis, cyanosis, unconsciousness, loss of reflexes, and death. Contact with eyes causes irritation and lachrymation. Can be absorbed through the skin and may produce severe skin lesions.
Fire Hazard
Special Hazards of Combustion Products: Irritating hydrogen chloride vapor may form in fire.
Potential Exposure
Tetrachloroethane is used as an intermediate in the trichloroethylene production from acetylene and as a solvent; as a dry cleaning agent; as a fumigant; in cement; and in lacquers. It is used in the manufacture of artificial silk, artificial leather, and artificial pearls. Recently, its use as a solvent has declined due to replacement by less toxic compounds. It is also used in the estimation of water content in tobacco and many drugs, and as a solvent for chromium chloride impregnation of furs.
Carcinogenicity
The EPA has classified this
material as “likely to be carcinogenic to humans” based on data from an oral cancer bioassay in male and female
Osborne–Mendel rats and B6C3F1 mice. In mice, a
significant increase in the incidence of hepatoceullar carcinomas
in both genders was observed. Male Osborne–Mendel
rats showed increased incidence of hepatocellular carcinomas,
which is a rare tumor in this strain.
The National Cancer Institute has included 1,1,2,2-
tetrachloroethane in their bioassay series using rats and mice.
Their summary states that the time-weighted average doses
(by gavage) were 108 and 62 mg/kg/day for male rats, 76 and
43 mg/kg/day for female rats, and 282 and 142 mg/kg/day for
all mice. There was a highly significant positive dose-related
trend in the incidence of hepatocellular carcinoma in mice of
both sexes. No statistically significant incidence of neoplastic
lesions was observed in male or female rats. However, two
hepatocellular carcinomas and one neoplastic nodule, which
are rare tumors in the male Osborne–Mendel rat, were
observed in high-dose males. Under the conditions of this
bioassay, orally administered 1,1,2,2-tetrachloroethane was
a liver carcinogen in B6C3Fl mice of both sexes.
The proposed metabolic pathway involves the production
of dichloroacetic acid, which was identified as the
major urinary metabolite in treated mice. Other pathways
involve formation of trichloroethylene via dehydrochlorination
or tetrachloroethylene via oxidation. Free radicals may
also be formed.
From the NCI study, a oral slope factor (OSF) of 0.2
per mg/kg/day was developed by the EPA. No inhalation unit
risk (IUR) was determined by the EPA because of absence of
data from inhalation exposure.
Shipping
UN1702 Tetrachloroethane or 1,1,2,2Tetrachloroe thane, Hazard Class: 6.1; Labels: 6.1Poisonous materials.
Purification Methods
Stir the ethane, on a steam-bath, with conc H2SO4 until a fresh portion of acid remains colourless. The organic phase is then separated, distilled in steam, dried (CaCl2 or K2CO3), and fractionally distilled in a vacuum. [Beilstein 1 IV 144.]
Incompatibilities
Violent reaction with chemically active metals; strong caustics; strong acids; especially fuming sulfuric acid. Degrades slowly when exposed to air. Attacks plastic and rubber.
Waste Disposal
Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal. Incineration, preferably after mixing with another combustible fuel. Care must be exercised to assure complete combustion to prevent the formation of phosgene. An acid scrubber is necessary to remove the halo acids produced.
1,1,2,2-Tetrachloroethane Preparation Products And Raw materials
Raw materials
Preparation Products
1of3
chevron_right| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| JSK Chemicals | +919879767970 | Gujarat, India | 3756 | 58 | Inquiry |
| RYZE CHEMIE | +91-9702966440 +91-9702966440 | Maharashtra, India | 335 | 58 | Inquiry |
| Titan Biotech Limited | 09711169006 | Delhi, India | 196 | 58 | Inquiry |
| Anand Agencies | 91-20-24454597 | Maharashtra, India | 2337 | 58 | Inquiry |
| Loba Chemie Pvt., Ltd. | 91-22-66636663 | Maharashtra, India | 766 | 58 | Inquiry |
| THOMAS BAKER (CHEMICALS) PVT. LTD. | +91-22-22811264 | New Delhi, India | 86 | 47 | Inquiry |
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6351 | 58 | Inquiry |
| A.J Chemicals | 91-9810153283 | New Delhi, India | 6124 | 58 | Inquiry |
| Maas Pharma Chemicals | +91-9958666224 | Delhi, India | 1607 | 58 | Inquiry |
| Otto Chemie Pvt. Ltd. | +91 9820041841 | Mumbai, India | 5873 | 58 | Inquiry |
| Supplier | Advantage |
|---|---|
| JSK Chemicals | 58 |
| RYZE CHEMIE | 58 |
| Titan Biotech Limited | 58 |
| Anand Agencies | 58 |
| Loba Chemie Pvt., Ltd. | 58 |
| THOMAS BAKER (CHEMICALS) PVT. LTD. | 47 |
| CLEARSYNTH LABS LTD. | 58 |
| A.J Chemicals | 58 |
| Maas Pharma Chemicals | 58 |
| Otto Chemie Pvt. Ltd. | 58 |
Related articles
- 1,1,2,2-Tetrachloroethane: Properties, Production process and Uses
- 1,1,2,2-Tetrachloroethane is used as an intermediate for trichloroethylene, tetrachloroethylene, pentachloroethane and hexachl....
- Mar 19,2024
79-34-5(1,1,2,2-Tetrachloroethane)Related Search:
1of4
chevron_right




