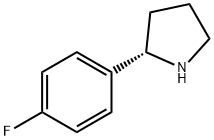
(S)-2-(4-Fluorophenyl)pyrrolidine
- CAS No.
- 298690-90-1
- Chemical Name:
- (S)-2-(4-Fluorophenyl)pyrrolidine
- Synonyms
- (S)-2-(4-Fluorophenyl)pyrrolidine;(2S)-2-(4-fluorophenyl)pyrrolidine;Pyrrolidine, 2-(4-fluorophenyl)-, (2S)-;(S)-2-(4-Fluorophenyl)pyrrolidine (S)-2-(4-Fluorophenyl)pyrrolidine
- CBNumber:
- CB9875223
- Molecular Formula:
- C10H12FN
- Molecular Weight:
- 165.21
- MOL File:
- 298690-90-1.mol
- MSDS File:
- SDS
- Modify Date:
- 2023/10/10 10:48:53
| Boiling point | 235.5±33.0 °C(Predicted) |
|---|---|
| Density | 1.078±0.06 g/cm3(Predicted) |
| storage temp. | under inert gas (nitrogen or Argon) at 2–8 °C |
| pka | 9.76±0.10(Predicted) |
| CAS DataBase Reference | 298690-90-1(CAS DataBase Reference) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H315-H319-H335 |
| Precautionary statements | P261-P305+P351+P338 |
| HS Code | 2933998090 |
(S)-2-(4-Fluorophenyl)pyrrolidine Chemical Properties,Uses,Production
Uses
(S)-2-(4-Fluorophenyl)pyrrolidine is a pyrrole compound and can be used in the synthesis of organic compounds.
Application
(S)-2-(4-Fluorophenyl)pyrrolidine, also known as amide 2-(4-fluorophenyl)-2-pyrrolidinone, is a drug that has been discovered but not yet clinically developed. It is a blocker of the atrial natriuretic peptide receptor.
Synthesis
The mixture of tert-butyl (4-(4-fluorophenyl)-4-oxobutyl)carbamate (0.2 mmol) and trifluoroacetic acid (equiv) in CH2Cl2 was stirred under nitrogen for 3 h. After all the acid and solvent had been removed under vacuum, the obtained substance was transferred to a nitrogen-filled glovebox and dissolved in a mixed solvent of toluene/THF (5:1, 1 mL). Ti(OiPr)4 (0.24 mmol), DABCO (0.04 mmol), Ir-L9 (0.002 mmol) and KI (0.04 mmol) were added subsequently to the above solution. The total solution was made to 3.0 mL. The resulting vial was transferred to an autoclave, which was charged with 50 atm of H2, and stirred at 50 °C for 13 h. The solution was quenched with aqueous sodium bicarbonate solution. The organic phase was concentratedand the metal complex was removed by using a short silica plug to give the (S)-2-(4-Fluorophenyl)pyrrolidine.
(S)-2-(4-Fluorophenyl)pyrrolidine Preparation Products And Raw materials
Raw materials
Preparation Products
(S)-2-(4-Fluorophenyl)pyrrolidine Suppliers
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6351 | 58 | Inquiry |
| A.J Chemicals | 91-9810153283 | New Delhi, India | 6124 | 58 | Inquiry |
| Tianjin Xinghan Biotechnology Co., Ltd | +8618698089028 | China | 2479 | 58 | Inquiry |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | China | 49391 | 58 | Inquiry |
| Guangzhou Yuheng Pharmaceutical Technology Co., Ltd | +8613580539051 | CHINA | 21149 | 58 | Inquiry |
| Zhengzhou Alfa Chemical Co.,Ltd | +8618530059196 | China | 12922 | 58 | Inquiry |
| Career Henan Chemica Co | +86-0371-86658258 +8613203830695 | China | 30253 | 58 | Inquiry |
| Finetech Industry Limited | +86-27-87465837 +8618971612321 | China | 9626 | 58 | Inquiry |
| Dayang Chem (Hangzhou) Co.,Ltd. | 571-88938639 +8617705817739 | China | 52861 | 58 | Inquiry |
| Golden Pharma Co., Limited | +undefined18958062155 | China | 5905 | 58 | Inquiry |
298690-90-1((S)-2-(4-Fluorophenyl)pyrrolidine)Related Search:
1of4
chevron_right




