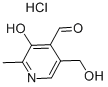
피리독살 하이드로클로라이드
|
|
피리독살 하이드로클로라이드 속성
- 녹는점
- 173 °C (dec.)(lit.)
- 저장 조건
- 2-8°C
- 용해도
- DMSO(약간 용해됨), 메탄올(약간 용해됨), 물(약간 용해됨)
- 물리적 상태
- 가루
- 색상
- 하얀색
- 생물학적 소스
- synthetic (Organic)
- 수용성
- 물과 에탄올에 용해됩니다.
- 감도
- Hygroscopic
- Merck
- 14,7978
- BRN
- 3656994
- InChI
- InChI=1S/C8H9NO3.ClH/c1-5-8(12)7(4-11)6(3-10)2-9-5;/h2,4,10,12H,3H2,1H3;1H
- InChIKey
- FCHXJFJNDJXENQ-UHFFFAOYSA-N
- SMILES
- C1(C=O)=C(O)C(=NC=C1CO)C.Cl
- CAS 데이터베이스
- 65-22-5(CAS DataBase Reference)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | Xi | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 20/21/22-36/37/38 | ||
| 안전지침서 | 24/25-22-36-26 | ||
| WGK 독일 | 2 | ||
| RTECS 번호 | UV1225000 | ||
| F 고인화성물질 | 8 | ||
| TSCA | Yes | ||
| HS 번호 | 29362500 |
피리독살 하이드로클로라이드 C화학적 특성, 용도, 생산
개요
Pyridoxal hydrochloride is a hydrochloride salt formed by combining pyridoxal with hydrochloric acid. It is also a derivative of pyridinium salt and vitamin B6. It has important roles in E. coli metabolites, Saccharomyces cerevisiae metabolites, cofactors, human metabolites and mouse metabolites. It can be used as a calibrator for liquid chromatography of food samples, a component of culture media for lactic acid bacteria (LAB) and foodborne pathogens (FBP) (component of arginine decarboxylase and lysine decarboxylase).화학적 성질
White to off-white crystalline powder용도
Pyridoxal hydrochloride is used in labeling of amino acids for their detection. It finds application in neurotransmitters, sphingolipids and aminolevulinic acid. It is also used as a nutritional agent. It is also applied in research studies like biochemical, drugs and in media supplements.정의
ChEBI: Pyridoxal hydrochloride is a hydrochloride obtained by combining pyridoxal with one molar equivalent of hydrochloric acid. It has a role as an Escherichia coli metabolite, a Saccharomyces cerevisiae metabolite, a cofactor, a human metabolite and a mouse metabolite. It is a hydrochloride, a pyridinium salt and a vitamin B6. It contains a pyridoxal(1+).일반 설명
Pyridoxal is a heterocyclic compound, weighing 167.2 Da. It is one of the natural forms of vitamin B6. Pyridoxal is found to be less stable than pyridoxine, hence heating might result in the loss of its action. Pyridoxal has a wide variety of sources and is present in both plants and animals. Pyridoxal serves as an efficient precursor for coenzymes : pyridoxal phosphate and pyridoxamine phosphate.Safety Profile
Poison by intramuscular, intravenous, and intraperitoneal routes. Moderately toxic by ingestion and subcutaneous routes. When heated to decomposition it emits very toxic fumes of NOx and HCl. See also ALDEHYDES.Synthesis
In the 25mL reaction flask with agitator and thermometer, add successively 0.085g (0.5mmol) 2,2,6,6-tetramethyl piperidine-nitrogen-oxyradical, 0.29g (2mmol) cupric bromide, 0.40g (5mmol) pyridine and 2.06g (10mmol) pyridoxinehydrochloride. Add water 5mL, temperature is controlled at 30 DEG C, opens and stirs.Then drip 2.0g (18mmol) 30% hydrogen peroxide, control temperature of reaction at 30~35 DEG C simultaneously.After dropwising, insulation continues to stir 1~2 hour at 30~35 DEG C, and gained reaction solution reaches 99% (liquid-phase condition: chromatographic column is XDB-G8 250 × 4.6 mm 5um through Liquid Detection pyridoxol transformation efficiency.Moving phase is methyl alcohol: buffer=15: 85; Buffer:0.04% sodium pentanesulfonate is adjusted PH to 3 with Glacial acetic acid; Detection wavelength is 284nm), reaction preference is 98%.In addition, get about 5mL reaction solution and directly go up silicagel column, eluent is methylene dichloride: methyl alcohol=20: 1, obtain final elutriant through gradient elution, and be concentrated into dryly, can obtain white solidpyridoxalhydrochloride, fusing point 164.1-164.8 DEG C, nuclear-magnetism characterizes as follows: 1hNMR (400MHz, DMSO), δ: 8.27 (s, 1H, CHO), 6.63 (d, 1H, CH), 5.00-5.16 (m, 2H, CH2), 2.62 (s, 3H, CH3).
Because pyridoxalhydrochloride is unstable, difficult separation, reaction solution then adds p-ethoxyaniline 1.37g (10mmol), treated yellow Schiff alkali (IX) 2.10g that obtains without separating.The total recovery of two-step reaction is 88%.
Purification Methods
Dissolve it in water and adjust the pH to 6 with NaOH. Set aside overnight to crystallise. The crystals are washed with cold water, dried in a vacuum desiccator over P2O5 and stored in a brown bottle at room temperature. The free base is then converted to the hydrochloride with one equivalent of HCl. [Fleck & Alberty J Phys Chem 66 1678 1962, Beilstein 21/13 V 44.]피리독살 하이드로클로라이드 준비 용품 및 원자재
원자재
준비 용품
피리독살 하이드로클로라이드 공급 업체
글로벌( 404)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| SHANGHAI SAGACHEM CO., LTD. | +86-02168404722 +86-13816115801 |
david_si@shjlchem.com | China | 197 | 58 |
| Shanghai Jinli Pharmaceutical Co.,Ltd | +86-21-57256005 +86-15921811235 |
chang@shjlchem.com | China | 20 | 58 |
| Hebei Mujin Biotechnology Co.,Ltd | +86 13288715578 +8613288715578 |
sales@hbmojin.com | China | 12814 | 58 |
| Wuhan Fortuna Chemical Co., Ltd | +86-027-59207850 |
info@fortunachem.com | China | 5969 | 58 |
| hebei hongtan Biotechnology Co., Ltd | +852-6619 3215 +86-17731935328 |
sales03@chemcn.cn | China | 970 | 58 |
| BINBO BIOLOGICAL CO.,LTD | +8618629063126 |
info@binbobiological.com | China | 449 | 58 |
| Shandong Deshang Chemical Co., Ltd. | +86-0531-8875-2665 +8613153039501 |
info@deshangchem.com | China | 662 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21629 | 55 |
| career henan chemical co | +86-0371-86658258 +8613203830695 |
sales@coreychem.com | China | 29860 | 58 |
| NINGBO INNO PHARMCHEM CO., LTD. | 13867897135 |
sales@nbinno.com | CHINA | 923 | 58 |