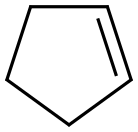
사이클로펜텐
|
|
사이클로펜텐 속성
- 녹는점
- −135 °C(lit.)
- 끓는 점
- 44-46 °C(lit.)
- 밀도
- 0.771 g/mL at 25 °C(lit.)
- 증기압
- 20.89 psi ( 55 °C)
- 굴절률
- n
20/D 1.421(lit.)
- 인화점
- <−30 °F
- 저장 조건
- 0-6°C
- 용해도
- 물: 25°C에서 용해성0.535g/L
- 물리적 상태
- 액체
- 색상
- 무색의
- Specific Gravity
- 0.771
- 수용성
- 섞일 수 없음
- 감도
- Air Sensitive
- BRN
- 635707
- Henry's Law Constant
- 6.3 x 10-2 atm?m3/mol at 25 °C (Hine and Mookerjee, 1975)
- 안정성
- 안정적인. 가연성이 높습니다. 강한 산화제와 호환되지 않습니다. 차갑게 보관하세요.
- InChIKey
- LPIQUOYDBNQMRZ-UHFFFAOYSA-N
- LogP
- 2.47 at 25℃
- CAS 데이터베이스
- 142-29-0(CAS DataBase Reference)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | F,Xn | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 11-21/22-36/37/38-65-67-52/53-38 | ||
| 안전지침서 | 9-16-26-33-36-62-61-36/37 | ||
| 유엔번호(UN No.) | UN 2246 3/PG 2 | ||
| WGK 독일 | 3 | ||
| RTECS 번호 | GY5950000 | ||
| F 고인화성물질 | 10-23 | ||
| 자연 발화 온도 | 743 °F | ||
| TSCA | Yes | ||
| 위험 등급 | 3 | ||
| 포장분류 | II | ||
| HS 번호 | 29021990 | ||
| 독성 | Acute oral LD50 for rats is 1,656 mg/kg (quoted, RTECS, 1985). | ||
| 기존화학 물질 | KE-09303 |
사이클로펜텐 C화학적 특성, 용도, 생산
화학적 성질
Cyclopentene is a highly flammable liquid with a low flash point. It reacts readily with oxidizing agents.물리적 성질
Clear, colorless, watery, very flammable liquid with a characteristic sweet, petroleum-like odor.용도
Cyclopentene is a cycloalkene that is cyclopentane having one endocyclic double bond. Vapors heavier than air. Inhalation of high concentrations may be narcotic. Used to make rubber and plastics. Neopentyl phosphine ligand catalyzed Heck coupling of cyclopentene has been reported. Mechanism of reaction of ground state oxygen atom with cyclopentene has been investigated. Homopolymerization of cyclopentene has been reported. Photocatalytic oxidation of cyclopentene over various titanium(IV) oxide catalyst has been reported. Cyclopentene was used to investigate the [2+2] cycloaddition of diamond (001) surfaces with alkene.정의
ChEBI: Cyclopentene is a cycloalkene that is cyclopentane having one endocyclic double bond.제조 방법
Cyclopentene is synthesized by selective hydrogenation of cyclopentadiene or by dehydration of cyclopentanol. It is produced industrially in large amounts by steam cracking of naphtha. Cyclopentene is present in coal tar, cigarette smoke, and automobile emissions.일반 설명
Cyclopentene appears as a colorless liquid. Less dense than water and insoluble in water. Flash point below 0°F. Vapors heavier than air. Inhalation of high concentrations may be narcotic. Used to make rubber and plastics.공기와 물의 반응
Highly flammable. Insoluble in water.반응 프로필
Cyclopentene may react vigorously with strong oxidizing agents. May react exothermically with reducing agents to release hydrogen gas. In the presence of various catalysts (such as acids) or initiators, may undergo exothermic addition polymerization reactions.건강위험
May be harmful by inhalation, ingestion, or skin absorption. May cause eye and skin irritation.화재위험
Special Hazards of Combustion Products: Vapor may travel considerable distance to a source of ignition and flashback. Explosion may occur under fire condition.Safety Profile
Moderately toxic by ingestion and skin contact. A very dangerous fire hazard when exposed to flame or heat; can react with oxidning materials. Keep away from heat and open flame. To fight fire, use foam, CO2, dry chemical.환경귀착
Biological. Cyclopentene may be oxidized by microbes to cyclopentanol, which may oxidize to cyclopentanone (Dugan, 1972).Photolytic. The following rate constants were reported for the reaction of cyclopentene with OH radicals in the atmosphere: 6.39 x 10-11 cm3/molecule?sec (Atkinson et al., 1983), 4.99 x 10-11 cm3/molecule?sec at 298 K (Rogers, 1989), 4.0 x 10-10 cm3/molecule?sec (Atkinson, 1990) and 6.70 x 10-11 cm3/molecule?sec (Sablji? and Güsten, 1990); with ozone in the atmosphere: 8.13 x 10-16 at 298 K (Japar et al., 1974) and 9.69 x 10-16 cm3/molecule?sec at 294 K (Adeniji et al., 1981); with NO3 in the atmosphere: 4.6 x 10-13 cm3/molecule?sec at 298 K (Atkinson, 1990) and 5.81 x 10-13 cm3/molecule?sec at 298 K (Sablji? and Güsten, 1990).
Chemical/Physical. Gaseous products formed from the reaction of cyclopentene with ozone were (% yield): formic acid, carbon monoxide, carbon dioxide, ethylene , formaldehyde, and butanal. Particulate products identified include succinic acid, glutaraldehyde, 5-oxopentanoic acid, and glutaric acid (Hatakeyama et al., 1987).
At elevated temperatures, rupture of the C-C bond occurs forming molecular hydrogen and cyclopentadiene (95% yield) as the principal products (Rice and Murphy, 1942).
Purification Methods
Free cyclopentene from hydroperoxide by refluxing with cupric stearate. Fractionally distil it from Na. It can be chromatographed on a Dowex 710-Chromosorb W GLC column. Methods for cyclohexene should be applicable here. Also, it has been washed with 1M NaOH solution followed by water. It was dried over anhydrous Na2SO4, distilled over powdered NaOH under nitrogen, and passed through neutral alumina before use [Woon et al. J Am Chem Soc 108 7990 1986]. It was distilled in a dry nitrogen atmosphere from powdered fused NaOH through a Vigreux column (p 11), and then passed through activated neutral alumina before use [Wong et al. J Am Chem Soc 109 3428 1987]. [Beilstein 5 IV 209.]사이클로펜텐 준비 용품 및 원자재
원자재
준비 용품
Ramipril
세바식산
2-Allyl-4-hydroxy-3-methyl-2-cyclopenten-1-one
LENAMPICILLIN
2-클로로페닐사이클로펜틸케톤
Ketamine hydrochloride
ROSAPROSTOL
Prostaglandin E1
3-Ethyl-2-hydroxy-2-cyclopenten-1-one
KETAMINE RELATED COMPOUND A (50 MG) (1 -[(2-CHLOROPHENYL)(METHYLIMINO)METHYL]CYLCOPENTA-NOL)
Cyclopentene oxide
시클로펜타디엔
CIS-1,2-CYCLOPENTANEDIOL
바이사이클로펜틸
Cyclopentanemethanol
이소프로필시클로펜탄
Silane, trichloro(1-methylethoxy)-
사이클로펜텐 공급 업체
글로벌( 310)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Hebei Chuanghai Biotechnology Co., Ltd | +86-15531157085 +86-15531157085 |
abby@chuanghaibio.com | China | 8808 | 58 |
| Hebei Mujin Biotechnology Co.,Ltd | +86 13288715578 +8613288715578 |
sales@hbmojin.com | China | 12814 | 58 |
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 |
sales1@chuanghaibio.com | China | 5870 | 58 |
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 |
info@fdachem.com | China | 20238 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21629 | 55 |
| Shanghai Time Chemicals CO., Ltd. | +86-021-57951555 +8617317452075 |
jack.li@time-chemicals.com | China | 1803 | 55 |
| Zhejiang ZETian Fine Chemicals Co. LTD | +8618957127338 |
stella@zetchem.com | China | 2136 | 58 |
| Kingfirst chemical (nanjing) co.,ltd | 86-25-84522992 |
marketing@kingfirstchem.com | CHINA | 95 | 58 |
| Wuhan Chemwonders Technology Inc. | 027-85778276 |
info@chemwonders.com | CHINA | 176 | 58 |
| Xiamen AmoyChem Co., Ltd | +86-86-5926051114 +8615060885618 |
sales@amoychem.com | China | 6383 | 58 |