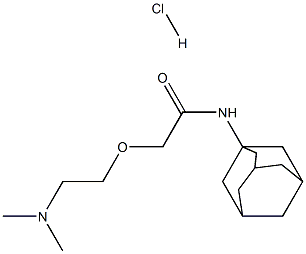
2-[2-(dimethylamino)ethoxy]-N-tricyclo[3.3.1.13,7]dec-1-ylacetamide monohydrochloride
|
|
2-[2-(dimethylamino)ethoxy]-N-tricyclo[3.3.1.13,7]dec-1-ylacetamide monohydrochloride 속성
- 녹는점
- 157-158°
- 물리적 상태
- Solid
- 색상
- White to off-white
안전
| 독성 | LD50 orally in rats: 630 mg/kg; i.v. in mice: 71.0 mg/kg (Peteri, Sterner) |
|---|
2-[2-(dimethylamino)ethoxy]-N-tricyclo[3.3.1.13,7]dec-1-ylacetamide monohydrochloride C화학적 특성, 용도, 생산
Originator
Viru-Merz,Merz,W. Germany,1973Manufacturing Process
Adamantane is first reacted with chloroacetyl chloride to give chloroacetylaminoadamantane. 2.3 g Na (0.1 g-atom) were dissolved in 75 ml dimethylamino-ethanol. Then the excess alcohol was distilled off completely and the sodium salt developed was dried in a vacuum. After drying, the salt was dissolved in about 200 ml xylene. To thissolution.22.8 g (0.1 mol) chloroacetylaminoadamantane were added, heated for 10 hours under reflux in a 250-ml round-bottomed flask with a reflux cooler, and the sodium chloride developed subsequently filtered off.Next the xylene was distilled away, the liquid residue dissolved in about 80 ml carbon tetrachloride and the hydrochloride precipitated through introduction of hydrochloric acid gas. The hydrochloride was dissolved in about 100 ml acetone and the solvent subsequently distilled away, whereby excess hydrochloric acid passed over with it. This operation was repeated until no excess acid was present.
A large excess of petroleum ether was added in a 500 ml three-necked flask provided with a stirrer and reflux cooler, to a concentrated acetonic solution of the hydrochloride and stirred for at least 1 hour, whereby the desired substance was deposited in a crystalline form. Finally, the substance was filtered away and dried in a desiccator. 14 g of the substance (15% of theory) were obtained.
Therapeutic Function
Antiviral2-[2-(dimethylamino)ethoxy]-N-tricyclo[3.3.1.13,7]dec-1-ylacetamide monohydrochloride 준비 용품 및 원자재
원자재
준비 용품
2-[2-(dimethylamino)ethoxy]-N-tricyclo[3.3.1.13,7]dec-1-ylacetamide monohydrochloride 공급 업체
글로벌( 10)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 |
marketing@targetmol.com | United States | 19892 | 58 |
| ShangHai Caerulum Pharma Discovery Co., Ltd. | 18149758185 |
sales-cpd@caerulumpharma.com | China | 3460 | 58 |
| Beijing Jin Ming Biotechnology Co., Ltd. | 010-60605840 18892239720 |
psaitong@jm-bio.com | China | 12308 | 58 |
| Shanghai Hongye Biotechnology Co. Ltd | 400-9205774 |
sales@glpbio.cn | China | 6870 | 58 |
| ChemeGen(Shanghai) Biotechnology Co.,Ltd. | 18818260767 |
sales@chemegen.com | China | 11289 | 58 |
| cjbscvictory | 13348960310 13348960310 |
3003867561@qq.com | China | 10011 | 58 |
| Beijing Jin Ming Biotechnology Co., Ltd. | 010-60605840 15801484223; |
psaitong@jm-bio.com | China | 29778 | 58 |
| TargetMol Chemicals Inc. | 4008200310 |
marketing@tsbiochem.com | China | 24017 | 58 |
| RD International Technology Co., Limited | 18024082417 |
market@ubiochem.com | China | 9282 | 58 |