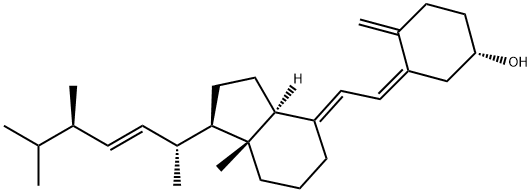
Synthesis of Vitamin D from β-Sitosterol
Jul 23,2024
The vitamin D family is known as vitamins D2 {ergocalciferol or (5Z,7E,22E)-(3S)-9,10-seco-5,7,10(19),22-ergostatetraen-3-ol}, D3 {cholecalciferol or (5Z,7E)-(3S)-9,10-seco-5,7,10(19)-cholestatrien-3-ol}, D4 {22,23-dihydroercalciol or (5Z,7E)-(3S)-9,10-seco-5,7,10(19)-ergostatrien-3-ol), D5 {(5Z,7E)-(3S)-9,10-seco-5,7,10(19)-stigmastatrien-3-ol}, D6 {(22E)-(24R)-ethyl-22,23-didehydrocalciol or (5Z,7E,22E)-(3S)-9,10-seco-5,7,10(19),22-stigmastatetraen-3-ol}, and D7 {(5Z,7E,24R)-(3S)-9,10-seco-5,7,10(19)-ergostatrien-3-ol} and contributes to important biological functions after converting to active forms.
Although these vitamin D analogs differ only in the structure of their side chains, they have different activities in vivo. For example, antirachitic activity using vitamins D2, D3, D4, D5, D6, and D7 showed a relative effect of 100:100:75:2:1:10, respectively. In addition, active forms of vitamin D3 are used as anti-osteoporosis drugs, but hypercalcemia due to excessive intake has been reported as a side effect. Active forms of vitamins D4 and D7 have been reported to be effective in improving this side effect.

Commercially available β-sitosterol was chosen as the starting material to synthesize various vitamin D analogs. It is not a synthetic product, but an extract from nature, and is a mixture of campesterol, stigmasterol, and brassicasterol according to the manufacturer. It was thought that vitamins D5, D7, D6, and D2 could be synthesized using these four starting materials, respectively. The conversion of phytosterol to vitamin D was performed with reference to previously established conditions.
First, the 3-position hydroxy group of commercially available β-sitosterol (1) was protected with an acetyl group (2), then it was brominated using N-bromosuccinimide (NBS), and 5,7-diene (3) was formed by debromination using tetrabutylammonium fluoride. Subsequently, the acetyl group at the 3-position hydroxy group was deprotected to synthesize provitamin D (4). It was converted to previtamin D (5) by irradiating with 280 nm UV light. Finally, previtamin D (5) was converted to vitamin D (6–9) by heating at 100 °C for 1 h. First, the synthesized vitamin D analogs (6–9) were separated from the raw material, previtamin D (5), using silica-gel column chromatography (ethyl acetate/n-hexane = 3:7). At this time, previtamin D (5) was not recovered. Next, in order to separate the synthesized vitamin D analogs, separation by HPLC was examined. When a 20 × 250 mm reverse phase C-18 HPLC column was used as a separation column and acetonitrile was used as a solvent, three large peaks were successfully separated. As a result of NMR and MS measurements, vitamin D2 (9, 1.75 mg, 67.6 min), a mixture (11.0 mg, 78.4 min) of vitamin D analogs, and vitamin D5 (6, 11.3 mg, 88.9 min) were found in ascending order of retention time.
References:
[1] SHIRO KOMBA W T Eiichi Kotake Nara. Simultaneous Synthesis of Vitamins D2, D4, D5, D6, and D7 from Commercially Available Phytosterol, β-Sitosterol, and Identification of Each Vitamin D by HSQC NMR.[J]. ACS Applied Electronic Materials, 2019. DOI:10.3390/metabo9060107.
- Related articles
- Related Qustion
1,7-Dimethylxanthine is a naturally occurring alkaloid compound that can enhance alertness and reduce drowsiness.....
Feb 27,2025APIAmmonia gas is easily compressed and forms a clear, colorless liquid under pressure. It is usually shipped as a compressed liquid in steel cylinders.....
Jul 23,2024Inorganic chemistryVitamin D2
50-14-6You may like
- Vitamin D2
-

- $10.00 / 1kg
- 2025-04-24
- CAS:50-14-6
- Min. Order: 1kg
- Purity: Food Grade
- Supply Ability: 10000
- Vitamin D2
-

- $0.00 / 1KG
- 2025-04-24
- CAS:50-14-6
- Min. Order: 1KG
- Purity: 99.4%
- Supply Ability: 500kg/month
- Vitamin D2; Secoergosta-5,7,10(19),22-tetraen-3-ol, (3beta,5Z,7E,22E)-
-

- $0.00 / 1g
- 2025-04-23
- CAS:50-14-6
- Min. Order: 10000g
- Purity: 99% HPLC
- Supply Ability: 1000kg