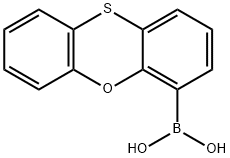
PHENOXATHIIN-4-BORONIC ACID
|
- ₹0
- Product name: PHENOXATHIIN-4-BORONIC ACID
- CAS: 100124-07-0
- MF: C12H9BO3S
- MW: 244.07
- EINECS:
- MDL Number:MFCD01605731
- Synonyms:4-Phenoxathinboronic acid;PHENOXANTHIIN-4-BORONIC ACID;Phenoxathiin-4-boronic acid,97%;4-Phenoxathiinylboronic acid, May contain varying amounts of anhydride, 97%;4-Phenoxathiineboronic acid;Phenoxathiin-4-boronic acid, 97% 100MG;phenoxathiin-4-ylboronic acid;4-PHENOXATHIINBORONIC ACID
| Manufacturer | Product number | Product description | Packaging | Price | Updated | Buy |
|---|
Properties
Melting point :162-167 °C
Boiling point :457.5±55.0 °C(Predicted)
Density :1.45±0.1 g/cm3(Predicted)
storage temp. :Keep in dark place,Inert atmosphere,Room temperature
pka :7.83±0.20(Predicted)
CAS DataBase Reference :100124-07-0(CAS DataBase Reference)
Boiling point :457.5±55.0 °C(Predicted)
Density :1.45±0.1 g/cm3(Predicted)
storage temp. :Keep in dark place,Inert atmosphere,Room temperature
pka :7.83±0.20(Predicted)
CAS DataBase Reference :100124-07-0(CAS DataBase Reference)
Safety Information
| Symbol(GHS): |

|
||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Signal word: | Warning | ||||||||||||||||||||||||||||
| Hazard statements: |
|
||||||||||||||||||||||||||||
| Precautionary statements: |
|
Description
phenoxathiin-4-boronic acid is used as a chemosensor due to its strong fluorescent when it binds to sugars.Related product price
- Phenothiazine
₹1395-178680.01 - 3-MERCAPTOPHENYLBORONIC ACID
₹7200-30300 - 128312-11-8
₹2620-13736.93






