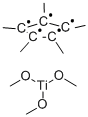
Trimethoxy(pentamethylcyclopentadienyl) titanium(IV)
|
- ₹0
- Product name: Trimethoxy(pentamethylcyclopentadienyl) titanium(IV)
- CAS: 123927-75-3
- MF: C10H15.C3H9O3Ti
- MW: 276.2
- EINECS:
- MDL Number:MFCD00269850
- Synonyms:PENTAMETHYLCYCLOPENTADIENE TITANIUM TRIMETHOXIDE;PENTAMETHYLCYCLOPENTADIENYLTITANIUM TRIMETHOXIDE;TRIMETHOXY(PENTAMETHYLCYCLOPENTADIENYL)TITANIUM(IV);Pentamethylcyclopentadienyltitaniumtrimethoxide,min.97%;PENTAMETHYLCYCLOPENTADIENYLTITANIUM(IV) TRIMETHOXIDE;Trimethoxy(pentamethylcyclopentadienyl)titanium(IV), 97+%;TRIMETHOXY(PENTAMETHYLCYCLOPENTADIENYL)&;PENTAMETHYLCYCLOPENTADIENYLTITANIUMTRIMETHOXIDE 97+%
| Manufacturer | Product number | Product description | Packaging | Price | Updated | Buy |
|---|
Properties
Boiling point :285 °C(lit.)
Density :1.081 g/mL at 25 °C(lit.)
refractive index :n20/D 1.554(lit.)
Flash point :141 °F
form :liquid
Specific Gravity :1.081
color :yellow
Hydrolytic Sensitivity :7: reacts slowly with moisture/water
Sensitive :Moisture Sensitive
CAS DataBase Reference :123927-75-3(CAS DataBase Reference)
Density :1.081 g/mL at 25 °C(lit.)
refractive index :n
Flash point :141 °F
form :liquid
Specific Gravity :1.081
color :yellow
Hydrolytic Sensitivity :7: reacts slowly with moisture/water
Sensitive :Moisture Sensitive
CAS DataBase Reference :123927-75-3(CAS DataBase Reference)
Safety Information
| Symbol(GHS): |

|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Signal word: | |||||||||||||||
| Hazard statements: |
|
||||||||||||||
| Precautionary statements: |
|
Description
Trimethoxy(pentamethylcyclopentadienyl)titanium(IV) is a highly insoluble thermally stable Titanium source suitable for glass, optic Ultra High PurityOxide Powderand ceramic applications. Oxide compounds are not conductive to electricity. However, certain perovskite structured oxides are electronically conductive finding application in the cathode of solid oxide fuel cells and oxygen generation systems. They are compounds containing at least one oxygen anion and one metallic cation. They are typically insoluble in aqueous solutions (water) and extremely stable making them useful in ceramic structures as simple as producing clay bowls to advanced electronics and in light weight structural components in aerospace and electrochemical applications such as fuel cells in which they exhibit ionic conductivity.More related product prices
4045-44-7 SODIUM CYCLOPENTADIENIDE TETRAPHENYLCYCLOPENTADIENONE METHYLCYCLOPENTADIENE DIMER TITANIUM TITANIUM Vinyltrimethoxysilane Titanium oxide (Trifluoromethoxy)benzene 12354-84-6 99604-67-8 92361-49-4 35344-11-7 75181-08-7 2290-65-5 TRIMETHYLSILYL ISOCYANATE Hexachlorocyclopentadiene 123927-75-3Related product price
- 4045-44-7
₹4540-31873.05 - SODIUM CYCLOPENTADIENIDE
₹8770-82843.73 - TETRAPHENYLCYCLOPENTADIENONE
₹1905.2-26683.63
Suppliers and manufacturers
Shanghai Daken Advanced Materials Co.,Ltd
Hangzhou FandaChem Co.,Ltd.
CONIER CHEM AND PHARMA LIMITED
Shaanxi Dideu Medichem Co. Ltd
Dayang Chem (Hangzhou) Co.,Ltd.
Hefei TNJ Chemical Industry Co.,Ltd.
PT CHEM GROUP LIMITED
GIHI CHEMICALS CO.,LIMITED
Shanghai Acmec Biochemical Technology Co., Ltd.
Aladdin Scientific






