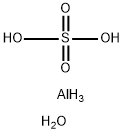
aluminium sulfate hexadecahydrate
|
- ₹0
- Product name: aluminium sulfate hexadecahydrate
- CAS: 16828-11-8
- MF: AlH7O5S
- MW: 146.09
- EINECS:605-511-8
- MDL Number:MFCD00149137
- Synonyms:ALUMINIUM SULPHATE;Aluminium sulfate 98-102 for analysis;ALUMINIUM SULFATE, DEHYDRATE;ALUM;LIQUID ALUM;aluminumsulfatehexahydrate ;sulfuricacid,aluminiumsalt(3:2),hexadecahydrate ;AluminiumSulphateFccFor16H2O
| Manufacturer | Product number | Product description | Packaging | Price | Updated | Buy |
|---|
Properties
Melting point :770 °C (dec.)(lit.)
Density :2.71 g/mL at 25 °C(lit.)
storage temp. :Sealed in dry,Room Temperature
LogP :-1.031 (est)
CAS DataBase Reference :16828-11-8(CAS DataBase Reference)
Density :2.71 g/mL at 25 °C(lit.)
storage temp. :Sealed in dry,Room Temperature
LogP :-1.031 (est)
CAS DataBase Reference :16828-11-8(CAS DataBase Reference)
Safety Information
| Symbol(GHS): |

|
|||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Signal word: | Danger | |||||||||||||||||||||
| Hazard statements: |
|
|||||||||||||||||||||
| Precautionary statements: |
|
Description
Aluminum Sulfate Hexadecahydrate is a moderately water and acid soluble Aluminum source for uses compatible with sulfates.Sulfate compounds are salts or esters of sulfuric acid formed by replacing one or both of the hydrogens with a metal. Most metal sulfate compounds are readily soluble in water for uses such as water treatment, unlike fluorides and oxides which tend to be insoluble. Organometallic forms are soluble in organic solutions and sometimes in both aqueous and organic solutions. Metallic ions can also be dispersed utilizing suspended or coated nanoparticles (See also application discussion at Nanotechnology Information and at Quantum Dots) and deposited utilizing sputtering targets and evaporation materials for uses such as solar energy materials and fuel cells.More related product prices
ALUMINUM SULFATE 7784-31-8 Sulfuric acid AMMONIUM ALUM Aluminium sulfate 7783-85-9 Aluminium sulfate hydrate 7783-83-7 7788-99-0 7784-24-9Related product price
- 7784-31-8
₹216-47121.23 - CARMINE
₹702-58774.5 - Sulfuric acid
₹520-212930.01






