Barium sulfite
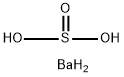
|
- ₹1179 - ₹4995
- Product name: Barium sulfite
- CAS: 7787-39-5
- MF: BaH4O3S
- MW: 221.42
- EINECS:232-112-2
- MDL Number:MFCD00040662
- Synonyms:BARIUM SULFITE;Sulfurous acid barium salt;barium sulphite
2 prices
Selected condition:
Brand
- ottokemi
Package
- 100gm
- 500gm
- Manufacturerottokemi
- Product number B 1368
- Product descriptionBarium sulphite
- Packaging500gm
- Price₹4995
- Updated2022-05-26
- Buy
- Manufacturerottokemi
- Product number B 1368
- Product descriptionBarium sulphite
- Packaging100gm
- Price₹1179
- Updated2022-05-26
- Buy
| Manufacturer | Product number | Product description | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|
| ottokemi | B 1368 | Barium sulphite | 500gm | ₹4995 | 2022-05-26 | Buy |
| ottokemi | B 1368 | Barium sulphite | 100gm | ₹1179 | 2022-05-26 | Buy |
Properties
Melting point :decomposed by heat [HAW93]
Density :4.440
solubility :insoluble in ethanol
form :white monoclinic crystals
color :white monoclinic crystals, crystalline
Water Solubility :0.0197g/100g H2O (20°C); 0.0018g/100g (80°C); soluble dilute HCl [KIR78] [HAW93]
Solubility Product Constant (Ksp) :pKsp: 9.3
Density :4.440
solubility :insoluble in ethanol
form :white monoclinic crystals
color :white monoclinic crystals, crystalline
Water Solubility :0.0197g/100g H2O (20°C); 0.0018g/100g (80°C); soluble dilute HCl [KIR78] [HAW93]
Solubility Product Constant (Ksp) :pKsp: 9.3
Safety Information
| Symbol(GHS): | ||||||||
|---|---|---|---|---|---|---|---|---|
| Signal word: | ||||||||
| Hazard statements: |
|
|||||||
| Precautionary statements: |
|
Description
Barium Sulfite can be prepared by reacting sodiumsulfite with bariumchloride:BaCl2+ Na2SO3→BaSO3+ 2NaCl
Barium Sulfite occurs as white, monoclinic crystals with a density of 4.44 g/cm3. Its solubility in water is low (0.001125 g/100 ml) and it is insoluble in ethanol. When heated, it decomposes at 480°C to the oxide and SO2 gas.
It has been used as a weighting material on oil-drilling rigs to prevent “blow out” during drilling operations. This salt is used for such purposes, presumably because of its ability to absorb SO2 and H2S gases trapped in the rocks as drilling proceeds. The demand for barium sulfite is low.
Barium sulfite is available in limited quantities, commercially.
More related product prices
7787-56-6 Strontium sulfate Magnesium sulfate Barium sulfate Ferrous sulfide BARIUM THIOSULFATERelated product price
- 7787-56-6
₹2779-27099 - Strontium sulfate
₹441-5337 - Magnesium sulfate
₹470-532980






