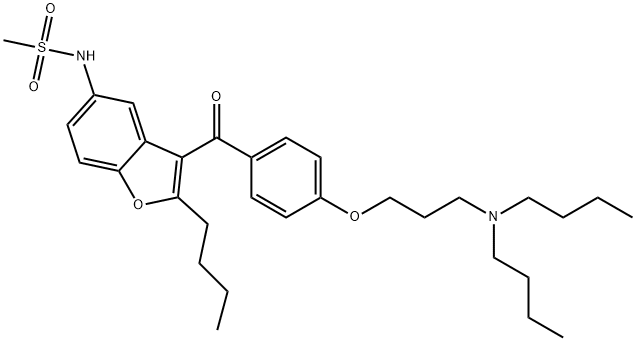
Dronedarone
|
- ₹0
- Product name: Dronedarone
- CAS: 141626-36-0
- MF: C31H44N2O5S
- MW: 556.76
- EINECS:
- MDL Number:MFCD00910331
- Synonyms:Dronedarone;N-(2-Butyl-3-(4-(3-(dibutylamino)propoxy)benzoyl)-5-benzofuranyl)methanesulfonamide;N-(2-butyl-3-(4-(3-(dibutylaMino)propoxy)benzoyl)benzofuran-5-yl)MethanesulfonaMide;MethanesulfonaMide,N-[2-butyl-3-[4-[3-(dibutylaMino)propoxy]benzoyl]-5-benzofuranyl]-;CS-294;Dronedarone (SR-33589);N-[2-butyl-3-[[4-[3-(dibutylamino)propoxy]phenyl]-oxomethyl]-5-benzofuranyl]methanesulfonamide;DRONEDARONE BASE
| Manufacturer | Product number | Product description | Packaging | Price | Updated | Buy |
|---|
Properties
Melting point :65.3°
Boiling point :683.9±65.0 °C(Predicted)
Density :1.143±0.06 g/cm3(Predicted)
storage temp. :2-8°C
solubility :≥27.84 mg/mL in DMSO; insoluble in H2O; ≥49.8 mg/mL in EtOH
form :solid
pka :7.40±0.30(Predicted)
color :White to off-white
CAS DataBase Reference :141626-36-0(CAS DataBase Reference)
Boiling point :683.9±65.0 °C(Predicted)
Density :1.143±0.06 g/cm3(Predicted)
storage temp. :2-8°C
solubility :≥27.84 mg/mL in DMSO; insoluble in H2O; ≥49.8 mg/mL in EtOH
form :solid
pka :7.40±0.30(Predicted)
color :White to off-white
CAS DataBase Reference :141626-36-0(CAS DataBase Reference)
Safety Information
| Symbol(GHS): |

|
||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Signal word: | Warning | ||||||||||||||||||||||||||||||||||||||||||
| Hazard statements: |
|
||||||||||||||||||||||||||||||||||||||||||
| Precautionary statements: |
|
Description
AF is the most common form of sustained cardiac arrhythmia, with an increasing prevalence in the aging population. AF accounts for 34.5% of arrhythmia-related hospital admissions in the United States. The most significant consequences of AF include congestive heart failure, a 5-fold increased risk of stroke, and increased rate of mortality. Although a 90% conversion rate from AF to normal sinus rhythm (NSR) can be achieved with electrical cardioversion, up to 70% of these patients require additional therapy with antiarrhythmic drugs in order to maintain NSR.Dronedarone, a close analog of amiodarone, is structurally modified to provide improved safety and pharmacokinetic profile. With the introduction of a sulfonamide group, dronedarone is less lipophilic, has lower tissue accumulation, and has a much shorter serum half-life (~24 h) compared with amiodarone. Additionally, dronedarone lacks the iodine moieties that are responsible for thyroid dysfunctions associated with amiodarone. Dronedarone is specifically indicated to reduce the risk of cardiovascular hospitalization in patients with paroxysmal or persistent AF or AFL, with a recent episode of AF/AFL and associated cardiovascular risk factors, who are in sinus rhythm or who will be cardioverted. Similar to amiodarone, dronedarone is a potent blocker of multiple ion currents (including the rapidly activating delayed-rectifier potassium current, the slowly activating delayed-rectifier potassium current, the inward rectifier potassium current, the acetylcholine-activated potassium current, peak sodium current, and L-type calcium current) and exhibits antiadrenergic effects. Overall, dronedarone was well tolerated. The most common side effects were gastrointestinal in nature and included nausea, vomiting, and diarrhea.
More related product prices
ILOPERIDONE Ranolazine Sotalol Propafenone 396-01-0 Dronedarone 52490-15-0 2-ButylbenzofuranRelated product price
- DRONEDARONE HYDROCHLORIDE
₹6300-38829.28 - ILOPERIDONE
₹2200-38547.83 - 396-01-0
₹4737-59477






