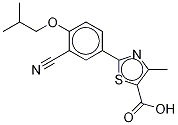
Febuxostat-d9
|
- ₹0
- Product name: Febuxostat-d9
- CAS: 1246819-50-0
- MF: C16H16N2O3S
- MW: 316.37484
- EINECS:
- MDL Number:MFCD21363615
- Synonyms:Febuxostat-d9;2-(3-Cyano-4-isobutyloxyphenyl-d9)-4-Methyl-5-thiazolecarboxylic Acid;2-[3-Cyano-4-(2-Methylpropoxy-d9)phenyl]-4-Methyl-5-thiazolecarboxylic Acid;TEI 6720-d9;TMX 67-d9;[2H9]-Febuxostat;Febuxostat-d9 (2-methylpropoxy-d9);Febuxostat D9Q: What is Febuxostat D9 Q: What is the CAS Number of Febuxostat D9 Q: What is the storage condition of Febuxostat D9 Q: What are the applications of Febuxostat D9
| Manufacturer | Product number | Product description | Packaging | Price | Updated | Buy |
|---|
Properties
Melting point :>201°C (dec.)
storage temp. :Refrigerator
solubility :Chloroform (Slightly), Methanol (Slightly)
form :Solid
color :White to Off-White
storage temp. :Refrigerator
solubility :Chloroform (Slightly), Methanol (Slightly)
form :Solid
color :White to Off-White
Safety Information
| Symbol(GHS): |

|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Signal word: | Warning | ||||||||||||||
| Hazard statements: |
|
||||||||||||||
| Precautionary statements: |
|
Description
Febuxostat-d9 is intended for use as an internal standard for the quantification of febuxostat by GC- or LC-MS. Febuxostat is an antihyperuricemic nonpurine inhibitor of both the oxidized and reduced forms of xanthine oxidase. It inhibits bovine milk xanthine oxidase as well as mouse and rat liver xanthine oxidase/xanthine dehydrogenase (IC50s = 1.4, 1.8, and 2.2 nM, respectively). It is 10-30 times more potent than the hypoxanthine analog allopurinol (; Kis = 0.7 nM and 0.7 μM, respectively). Febuxostat decreases the serum level of urate in a potassium oxonate rat model of hyperuricemia (ED50 = 1.5 mg/kg). It reduces hepatic macrovesicular steatosis in mice fed a high-fat diet containing trans fatty acids when administered at a dose of 1 mg/kg per day. Febuxostat (0.75 mg/kg) also increases CNS expression of glutamate oxaloacetate transaminase 2 (GOT2) and improves neurological symptoms in a mouse model of secondary progressive experimental autoimmune encephalomyelitis (EAE). Formulations containing febuxostat have been used in the treatment of symptomatic hyperuricemia in patients with gout.More related product prices
Febuxostat ETHYL 2-(3-FORMYL-4-ISOBUTOXYPHENYL)-4-METHYLTHIAZOLE-5-CARBOXYLATE ethyl 2-(3-formyl-4-hydroxyphenyl)-4-methyl thiazole-5-carboxylate Ethyl 2-(3-cyano-4-isobutoxyphenyl)-4-methyl-5-thiazolecarboxylate 2-[3-(AMinocarbonyl)-4-(2-Methylpropoxy)phenyl]-4-Methyl-5-thiazolecarboxylic Acid 2-[3-Carboxy-4-(2-Methylpropoxy)phenyl]-4-Methyl-5-thiazolecarboxylic Acid 2-HYDROXY-THIOBENZAMIDE 4-ISOBUTOXY-BENZALDEHYDE Febuxostat-d7 31643-49-9Related product price
- Febuxostat
₹9600-20448.43 - ethyl 2-(3-formyl-4-hydroxyphenyl)-4-methyl thiazole-5-carboxylate
₹2400-9200 - Ethyl 2-(3-cyano-4-isobutoxyphenyl)-4-methyl-5-thiazolecarboxylate
₹3200-12000






