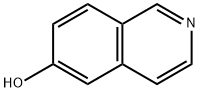
Isoquinolin-6-ol
|
- ₹0
- Product name: Isoquinolin-6-ol
- CAS: 7651-82-3
- MF: C9H7NO
- MW: 145.16
- EINECS:231-612-8
- MDL Number:MFCD04114860
- Synonyms:2H-Isoquinolin-6-one;6-HYDROXYISOQUINOLINE;isoquinolin-6-ol ;6-Isoquinolinol;Einecs 231-612-8;Isoquinolin-6-ol, 6-Hydroxy-2-azanaphthalene;6-Hydroxyisoquinoline96%;Isoquinolin-6-ol ISO 9001:2015 REACH
| Manufacturer | Product number | Product description | Packaging | Price | Updated | Buy |
|---|
Properties
Melting point :220 °C
Boiling point :332.1±15.0 °C(Predicted)
Density :1.260±0.06 g/cm3(Predicted)
storage temp. :2-8°C
form :solid
pka :9.15±0.10(Predicted)
color :Pale yellow
CAS DataBase Reference :7651-82-3(CAS DataBase Reference)
Boiling point :332.1±15.0 °C(Predicted)
Density :1.260±0.06 g/cm3(Predicted)
storage temp. :2-8°C
form :solid
pka :9.15±0.10(Predicted)
color :Pale yellow
CAS DataBase Reference :7651-82-3(CAS DataBase Reference)
Safety Information
| Symbol(GHS): |

|
||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Signal word: | Warning | ||||||||||||||||||||||||||||
| Hazard statements: |
|
||||||||||||||||||||||||||||
| Precautionary statements: |
|
Description
6-Hydroxyisoquinoline is used in proteomics research such as in preparation of isoquinoline derivatives which act as protease inhibitors against trypsin. It was also used as a reagent in the study of a novel series of compounds that act as Wnt signaling pathway inhibitors and obtained by scaffold hybridization strategy from two known porcupine inhibitor classes.More related product prices
4-Hydroxybenzoic acid 10-Hydroxycamptothecin 1,2,3,4-TETRAHYDROISOQUINOLINE 4-Methoxyphenol Isoquinoline Glycolic acid Hydroxyapatite 6-hydroxyquinoline-3-carboxylic acid 6-Hydroxyquinoxaline 6-Hydroxyindazole 6-Hydroxyindole 6299-87-2 3-Hydroxyisoquinoline Papaverine hydrochlorideRelated product price
- 4-Hydroxybenzoic acid
₹261-20697.4 - 10-Hydroxycamptothecin
₹14200-44804.68 - 1,2,3,4-TETRAHYDROISOQUINOLINE
₹2738.73-26400






