Relebactam
|
- ₹0
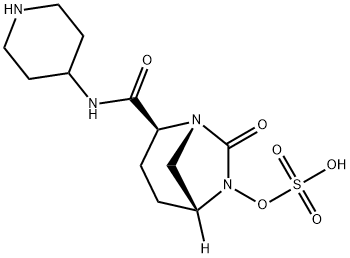
- Product name: Relebactam
- CAS: 1174018-99-5
- MF: C12H20N4O6S
- MW: 348.38
- EINECS:
- MDL Number:MFCD28502833
- Synonyms:Sulfuric acid Mono-[7-oxo-2-(piperidin-4-ylcarbaMoyl)-1,6-diaza-bicyclo[3.2.1]oct-6-yl] ester;Sulfuric acid mono[(1R,2S,5R)-7-oxo-2-[(4-piperidinylamino)carbonyl]-1,6-diazabicyclo[3.2.1]oct-6-yl] ester;MK-7655(Relebactam);RELEBACTAM;MK7655;(2S,5R)-sulfuric acid mono{[(4-aminopiperidin-4-yl)carbonyl]-7-oxo-1,6-diazabicyclo[3.2.1]oct-6-yl}ester;(2S,5R)-7-oxo-2-(piperidin-1-ium-4-ylcarbamoyl)-1,6-diazabicyclo[3.2.1]octan-6-yl sulfate;Relebactam(MK-7655)
| Manufacturer | Product number | Product description | Packaging | Price | Updated | Buy |
|---|
Properties
Melting point :>252°C (dec.)
Density :1.59
storage temp. :Hygroscopic, -20°C Freezer, Under inert atmosphere
solubility :DMSO (Slightly), Water (Slightly)
pka :-4.59±0.18(Predicted)
form :Solid
color :Off-White to Light Yellow
Stability :Hygroscopic
InChI :InChI=1S/C12H20N4O6S/c17-11(14-8-3-5-13-6-4-8)10-2-1-9-7-15(10)12(18)16(9)22-23(19,20)21/h8-10,13H,1-7H2,(H,14,17)(H,19,20,21)/t9-,10+/m1/s1
InChIKey :SMOBCLHAZXOKDQ-ZJUUUORDSA-N
SMILES :S(O)(ON1C(=O)[N@@]2C[C@@]1([H])CC[C@H]2C(NC1CCNCC1)=O)(=O)=O
Density :1.59
storage temp. :Hygroscopic, -20°C Freezer, Under inert atmosphere
solubility :DMSO (Slightly), Water (Slightly)
pka :-4.59±0.18(Predicted)
form :Solid
color :Off-White to Light Yellow
Stability :Hygroscopic
InChI :InChI=1S/C12H20N4O6S/c17-11(14-8-3-5-13-6-4-8)10-2-1-9-7-15(10)12(18)16(9)22-23(19,20)21/h8-10,13H,1-7H2,(H,14,17)(H,19,20,21)/t9-,10+/m1/s1
InChIKey :SMOBCLHAZXOKDQ-ZJUUUORDSA-N
SMILES :S(O)(ON1C(=O)[N@@]2C[C@@]1([H])CC[C@H]2C(NC1CCNCC1)=O)(=O)=O
Safety Information
| Symbol(GHS): | ||||||||
|---|---|---|---|---|---|---|---|---|
| Signal word: | ||||||||
| Hazard statements: |
|
|||||||
| Precautionary statements: |
|
Description
Relebactam (formerly known as MK-7655) is a novel,intravenous,class A and class C B-lactamase inhibitor and is currently under evaluation in combination with imipenem/cilastatin for the treatment of resistant Gram-negative infections.In vitro studies demonstrated that relebactam restored imipenem's activity against KPC-producing Enterobacteriacae,lowering imipenem MICs from 16-64 to 0.12-1mg/L at a concentration of 4 mg/L. Moreover, relebactam is able to lower imipenem MICs for P. aeruginosa,particularly in strains with depressed OprD expression and increased AmpC expression.Conversely, the addition of relebactam to imipenem does not seem to provide any adjunctive benefit against A.baumanii or S.maltophilia or against MBL-producing Enterobacteriacae.A non-inferiority, Phase 3 trial evaluating the efficacy and safety of imipenem/relebactam compared to piperacillin/tazobactam for the treatment of HAP and VAP(ClinicalTrials.gov Identifier: NCT02493764) is currently recruiting. A Phase 3 study evaluating the efficacy and safety of imipenem/relebactam (200/100-500/250 mg depending on renal function) compared to colistimethate sodium plus imipenem/cilastatin for the treatment of imipenem-resistant bacterial infections,including HAP, VAP, cIAIs and cUTIs, has recently been completed and results are pending(ClinicalTrials.gov Identifier: NCTO2452047).In Phase 2 trials, imipenem/relebactam was well tolerated, with diarrhea, nausea, vomiting and headache being the most commonly reported adverse events.More related product prices
Ammonium hydrogen sulfate N-Methyl-4-(methylamino)picolinamide 2-Pyridinecarboxamide,N-methyl-(9CI)Related product price
- Ammonium hydrogen sulfate
₹3591-73947
Suppliers and manufacturers
career henan chemical co
CLEARSYNTH LABS LTD.
Heading (Nanjing)Pharmtechnologies Co., Ltd.
ATK CHEMICAL COMPANY LIMITED
BOC Sciences
Chongqing Chemdad Co., Ltd
Zhejiang J&C Biological Technology Co.,Limited
ChemExpress
InvivoChem
₹1.00 /1kg
Sulfuric acid Mono-[7-oxo-2-(piperidin-4-ylcarbaMoyl)-1,6-diaza-bicyclo[3.2.1]oct-6-yl] ester CAS:1174018-99-5
Sulfuric acid Mono-[7-oxo-2-(piperidin-4-ylcarbaMoyl)-1,6-diaza-bicyclo[3.2.1]oct-6-yl] ester CAS:1174018-99-5






