Simeprevir
|
- ₹0
- Product name: Simeprevir
- CAS: 923604-59-5
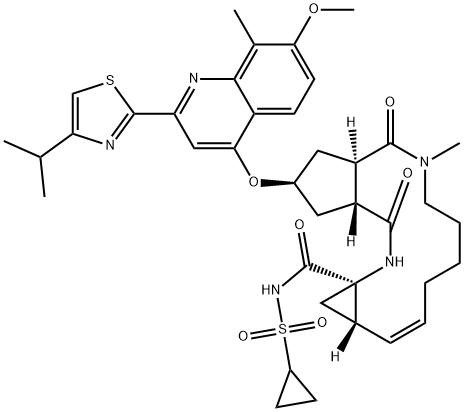
- MF: C38H47N5O7S2
- MW: 749.94
- EINECS:689-150-1
- MDL Number:MFCD25563225
- Synonyms:(2R,3aR,10Z,11aS,12aR,14aR)-N-(Cyclopropylsulfonyl)-2,3,3a,4,5,6,7,8,9,11a,12,13,14,14a-tetradecahydro-2-[[7-methoxy-8-methyl-2-[4-(1-methylethyl)-2-thiazolyl]-4-quinolinyl]oxy]-5-methyl-4,14-dioxocyclopenta[c]cyclopropa[g][1,6]diazacyclotetradecine-12a(1H)-carboxamide;TMC 435350;SiMeprevir;TMC435,TMC435350,TMC-435350;(2R,3aR,10Z,11aS,12aR,14aR)-N-(Cyclopropylsulfonyl)-2,3,3a,4,5,6,7,8,9,11a,12,13, 14,14a-tetradecahydro-2-[[7-;siMeprevir/TMC435;(2R,3aR,10Z,11aS,12aR,14aR)-N-(Cyclopropylsulfonyl)-2,3,3a,4,5,6,7,8,9,11a,12,13,14,14a-tetradecahydro-2-[[7-Methoxy-8-Methyl-2-[4-(1-Methylethyl)-2-thiazolyl]-4-quinolinyl]oxy]-5-Methyl-4,14-dioxocyclopenta[c]cyclopropa[g][1,6]diazacyclotetradecine-12a(1;Simeprevir(TMC 435350)
| Manufacturer | Product number | Product description | Packaging | Price | Updated | Buy |
|---|
Properties
Density :1.38
storage temp. :Store at -20°C
solubility :insoluble in H2O; insoluble in EtOH; ≥18.75 mg/mL in DMSO
form :solid
pka :4.47±0.40(Predicted)
color :White to off-white
storage temp. :Store at -20°C
solubility :insoluble in H2O; insoluble in EtOH; ≥18.75 mg/mL in DMSO
form :solid
pka :4.47±0.40(Predicted)
color :White to off-white
Safety Information
| Symbol(GHS): |

|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Signal word: | Warning | ||||||||||||||
| Hazard statements: |
|
||||||||||||||
| Precautionary statements: |
|
Description
In September 2013, simeprevir (also known as TMC435) was approved in Japan (trade name Sovriad?) for the treatment of genotype 1 hepatitis C virus (HCV) infection in combination with pegylated interferon and ribavirin (PR). Simeprevir was approved for the same indication in November 2013 in the United States (trade name Olysio?) and Canada (trade name Galexos?). Simeprevir is the third HCV PI to receive approval and was discovered from an effort to optimize a novel series of cyclopentane-core macrocyclic HCV PIs. Unlike the earlier PIs, simeprevir does not rely on formation of a covalent intermediate to inhibit the enzyme, but instead gains binding affinity through a large P2 quinoline substituent that occupies an extended S2 subsite of HCV protease by induced fit. This pocket is not occupied by inhibitors such as telaprevir and boceprevir. Other key features of simeprevir are truncation of the P3 capping group (the N-methyl amide), use of an acylsulfonamide as an acid isostere, and incorporation of an isopropylthiazole group to give improved permeability. Simeprevir is a potent NS3/4A PI (Ki=0.36 nM), with antiviral activity in the HCV replicon assay (genotype 1b EC50=7.8 nM; genotype 1a EC50=28.4 nM). It is 25-fold less potent against HCV genotype 2, >1000 less potent for HCV genotype 3, but has 3-fold better potency for HCV genotype 4.Related product price
- Atazanavir
₹12448.75-50152.23 - 4-[[6-amino-5-bromo-2-[(4-cyanophenyl)amino]-4-pyrimidinyl]oxy]-3, 5 –dimethylbenzonitrile
₹11517.8 - Darunavir
₹11420.38-46515.03






