Sodium antimonate
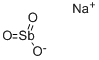
|
- ₹0
- Product name: Sodium antimonate
- CAS: 15432-85-6
- MF: Na.O3Sb
- MW: 192.75
- EINECS:239-444-7
- MDL Number:MFCD00058775
- Synonyms:SODIUM ANTIMONATE, 99.9%;Sodiumantimonate,min.95%;Natriumantimonat;Sodium antimonate trihydrate, 98+%;sodium antimonate(V);SODIUM ANTIMONATE;SODIUM METAANTIMONATE;Sodium antimonate trihydrate
| Manufacturer | Product number | Product description | Packaging | Price | Updated | Buy |
|---|
Properties
Melting point :>375 °C(lit.)
Density :3.7 g/mL at 25 °C(lit.)
Water Solubility :slightly soluble
Solubility Product Constant (Ksp) :pKsp: 7.4
Exposure limits :ACGIH: TWA 0.5 mg/m3
NIOSH: IDLH 50 mg/m3; TWA 0.5 mg/m3 Stability :Stable. Incompatible with strong oxidizing agents, strong acids, strong bases.
CAS DataBase Reference :15432-85-6(CAS DataBase Reference)
EPA Substance Registry System :Antimonate (SbO31-), sodium (15432-85-6)
Density :3.7 g/mL at 25 °C(lit.)
Water Solubility :slightly soluble
Solubility Product Constant (Ksp) :pKsp: 7.4
Exposure limits :ACGIH: TWA 0.5 mg/m3
NIOSH: IDLH 50 mg/m3; TWA 0.5 mg/m3 Stability :Stable. Incompatible with strong oxidizing agents, strong acids, strong bases.
CAS DataBase Reference :15432-85-6(CAS DataBase Reference)
EPA Substance Registry System :Antimonate (SbO31-), sodium (15432-85-6)
Safety Information
| Symbol(GHS): |
 
|
||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Signal word: | Warning | ||||||||||||||||||||||||||||
| Hazard statements: |
|
||||||||||||||||||||||||||||
| Precautionary statements: |
|
Description
A fine powder of sodium antimonate trihydrate, a slightly soluble salt of the highest available was used to prepare a dilute solution of sodium antimonate. In polyol method it is found that the ferrite is impossibly gained when diethylene glycol is replaced by ethylene glycol, or anhydrous sodium acetate by acetic acid sodium salt trihydrate.More related product prices
Sodium acetate Sodium silicate Diclofenac sodium Sodium sulfate Sodium percarbonate Trisodium citrate dihydrate Sodium chloride Sodium benzoate Sodium bicarbonate Sodium carbonate Sodium hydroxide Sodium formate Sodium citrate 16925-25-0 Sodium chlorite Sodium chlorate Sodium antimonateRelated product price
- Sodium acetate
₹310-188090 - Sodium silicate
₹1044-178140 - Diclofenac sodium
₹3439-52468.78
Suppliers and manufacturers
Chemico Chemicals Pvt Ltd
Clariant Chemicals India Limited
Lara Industries Private Limited
Jalan Slik Mill Private Limited
CHEMSWORTH






