neoantimycin
|
- ₹0
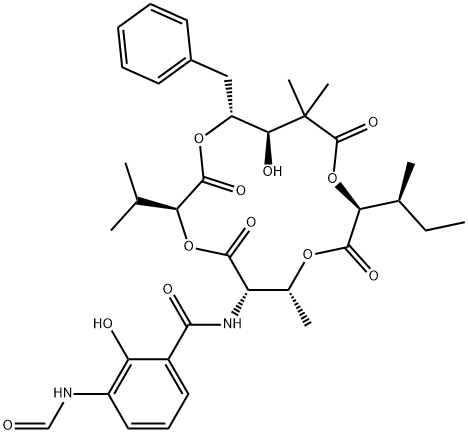
- Product name: neoantimycin
- CAS: 22862-63-1
- MF: C36H46N2O12
- MW: 698.76
- EINECS:
- MDL Number:MFCD11111613
- Synonyms:neoantimycin;N-(15-Benzyl-10-sec-butyl-14-hydroxy-3-isopropyl-7,13,13-trimethyl-2,5,9,12-tetraoxo-1,4,8,11-tetraoxacyclopentadeca-6-yl)-3-formylamino-2-hydroxybenzamide;Benzamide, 3-(formylamino)-2-hydroxy-N-[(3S,6S,7R,10S,14R,15R)-14-hydroxy-7,13,13-trimethyl-3-(1-methylethyl)-10-[(1S)-1-methylpropyl]-2,5,9,12-tetraoxo-15-(phenylmethyl)-1,4,8,11-tetraoxacyclopentadec-6-yl]-
| Manufacturer | Product number | Product description | Packaging | Price | Updated | Buy |
|---|
Properties
Boiling point :930.3±65.0 °C(Predicted)
Density :1.29±0.1 g/cm3(Predicted)
storage temp. :Store at -20°C
solubility :DMF: soluble; DMSO: soluble; Ethanol: soluble; Methanol: soluble
form :White to off-white solid.
pka :7.24±0.50(Predicted)
Density :1.29±0.1 g/cm3(Predicted)
storage temp. :Store at -20°C
solubility :DMF: soluble; DMSO: soluble; Ethanol: soluble; Methanol: soluble
form :White to off-white solid.
pka :7.24±0.50(Predicted)
Safety Information
| Symbol(GHS): | ||||||||
|---|---|---|---|---|---|---|---|---|
| Signal word: | ||||||||
| Hazard statements: |
|
|||||||
| Precautionary statements: |
|
Description
Neoantimycin is a macrocyclic antibiotic isolated from Streptomyces bacteria. The mechanism of action of Neoantimycin is undescribed in the literature. A closely related compound described as an oxidized-type neoantimycin, prunustatin A, is demonstrated to downregulate grp78 transcription and suppress the expression of the GRP 78 molecular chaperone. This suppression affects the role of GRP 78 in promoting protein folding at the endoplasmic reticulum (ER), and suppresses the pro-resistance enhancement of ER stress response by chemotherapy-resistant tumors. The close structural kinship between prunustatin A and Neoantimycin suggests a similar mechanism of action for Neoantimycin toxicity.Related product price
- FEMA 2871
₹1700-92261.48 - Butyl butyryllactate
₹2900-63315.43
Suppliers and manufacturers
CLEARSYNTH LABS LTD.
BOC Sciences
Shaanxi Cuikang Pharmaceutical Technology Co., Ltd
Aladdin Scientific
Shanghai Aladdin Bio-Chem Technology Co.,LTD
Beijing Lebo Biotechnology Co., Ltd.
Zhejiang Huida Biotech Co., LTD
Zhejiang Huida Biotech Co., LTD
Cayman Chemical Company
Shanghai EFE Biological Technology Co., Ltd.






