
2-(4-Nitrophenyl)thiophene synthesis
- Product Name:2-(4-Nitrophenyl)thiophene
- CAS Number:59156-21-7
- Molecular formula:C10H7NO2S
- Molecular Weight:205.23
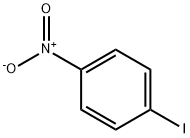
636-98-6
18 suppliers
$17.00/5g
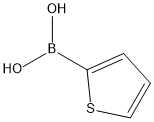
6165-68-0
384 suppliers
$14.00/5g

59156-21-7
21 suppliers
$106.00/25mg
Yield:59156-21-7 56%
Reaction Conditions:
with tetrakis(triphenylphosphine) palladium(0);N-phenylpicolinamide;copper in tetrahydrofuran at 20;Inert atmosphere;Suzuki Coupling;
Steps:
2-[4 - nitrophenyl] thiophene:
A two neck round bottom flask,equipped with a magnetic stir bar and a reflux condenser was flamed outunder a dry nitrogen purge. Upon cooling, the flask was charged with249.2 mg (1.0 equiv, 1.00 mmole) 4-nitro - 1 - iodobenzene, 396.8 mg (2.0 equiv.) NPPA ligand, 509.9 mg (8.0 equiv.) activated copper bronze, 140.9 mg (1.1 equiv.) 2-thiopheneboronic acid, and 116.5 mg (0.1 equiv.) tetrakis triphenyl phosphine palladium (0). 20.0 mLdry and degassed THF was added by syringe. Stirring was commenced for 24 hours at roomtemperature. The dark - brown reaction mixture was filtered through “celite.” The “celite” pad waswashed 4 X with 25 mL portions of ethyl acetate. The dark filtrate was washed 3 X 45 mL portionssaturated aqueous CuSO4. The organic extracts were washed with 50 mL brine, and filtered throughfluted filter paper and the filtrate concentrated by rotary evaporation to afford 815.2 mg of a dark redbrownoil. Prolonged extraction of this residue with several portions of hexanes followed by removalof the hexanes by rotary evaporation gave a residue which was subjected to flash chromatography onsilica 60 (230-400 mesh) eluted with 9:1 hexanes:ethyl acetate gave 116.4 mg (56 %) of 2-[4 -nitrophenyl] thiophene as a yellow solid. After obtaining proton and carbon -13 NMR spectra, thesolid darkened. The solid was stirred with hexanes for a prolonged period of time and the mixturecentrifuged. The supernatant was filtered through a cotton plug and the filtrate concentrated by rotaryevaporation to yield 23.3 mg of a solid for FT-IR. IR (ZnSe) ν (cm-1): 712 (w), 748 (w), 1054 (w),1105 (m), 1182 (w), 1212(w), 1316(w), 1419 (w), 1506 (w), 1590 (w), 2581(w), 2921(w), 3071(w),3103(w); 1H NMR (300 MHz, CDCl3) δ 7.16 (t, J = 5.1, 1.2, 1H), 7.44 - 7.49 (m, 2H), 7.75 (d, J = 9,2H), 8.24 (d, J = 9, 2H); 13C NMR (75 MHz, CDCl3) δ 124.3, 125.6, 125.9, 127.6, 128.6, 140.5,141.5, 146.5.
References:
Damkaci, Fehmi;Altay, Esra;Waldron, Matthew;Knopp, Michael A.;Snow, David;Massaro, Nicholas [Tetrahedron Letters,2014,vol. 55,# 3,p. 690 - 693] Location in patent:supporting information

6165-68-0
384 suppliers
$14.00/5g
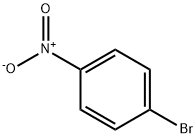
586-78-7
9 suppliers
$14.00/5g

59156-21-7
21 suppliers
$106.00/25mg

6165-68-0
384 suppliers
$14.00/5g

100-00-5
8 suppliers
$14.00/25g

59156-21-7
21 suppliers
$106.00/25mg
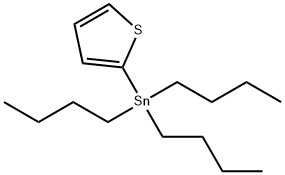
54663-78-4
138 suppliers
$25.00/5g

586-78-7
9 suppliers
$14.00/5g

59156-21-7
21 suppliers
$106.00/25mg
188290-36-0
1 suppliers
inquiry

100-01-6
10 suppliers
$11.19/25G

59156-21-7
21 suppliers
$106.00/25mg