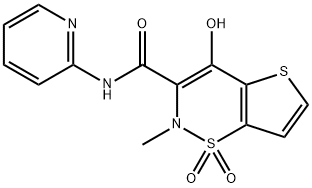
Tenoxicam synthesis
- Product Name:Tenoxicam
- CAS Number:59804-37-4
- Molecular formula:C13H11N3O4S2
- Molecular Weight:337.37
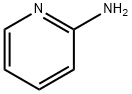
504-29-0
556 suppliers
$5.00/5 g
![METHYL 2-METHYL-4-HYDROXY-2H-THIENO[2,3-E]-1,2-THIAZINE-3-CARBOXYLATE-1,1-DIOXIDE](/CAS/GIF/59804-25-0.gif)
59804-25-0
136 suppliers
$250.00/250mg

59804-37-4
323 suppliers
$5.00/250mg
Yield:59804-37-4 76.9%
Reaction Conditions:
in 5,5-dimethyl-1,3-cyclohexadiene for 6 h;Reflux;Concentration;
Steps:
1.4 4. tenoxicam
5.4 g of TNXK-3, 300 ml xylene and 1.4g 2-aminopyridine in xylene solution 2.8 ml heating to reflux. After stirring reaction 6 hours, cooling. the majority of the solvent is removed under reduced pressure, cooling, filtering, the filter cake by adding 15 ml in water, adding sodium hydroxide 1 g and methanol 55 ml, heating the solvent, used to decolorize with active carbon, the filtrate is adjusted to PH hydrochloric acid 3, is cooled and is filtered, the filter cake is benzodioxane recrystallized, to get the yellow solid 4.2 g, yield 76.9%.
References:
Jiangxi Yong Tong Technology Co., Ltd.;Liu Zhongchun CN107141308, 2017, A Location in patent:Paragraph 0056-0060; 0066; 0070; 0076; 0080; 0086; 0090

504-29-0
556 suppliers
$5.00/5 g
![METHYL 4-HYDROXY-2H-THIENO[2,3-E]-1,2-THIAZINE-3-CARBOXYLATE-1,1-DIOXIDE](/CAS/GIF/98827-44-2.gif)
98827-44-2
81 suppliers
$126.00/500mg
616-38-6
697 suppliers
$5.00/5 g

59804-37-4
323 suppliers
$5.00/250mg
59337-92-7
237 suppliers
$10.00/5g

59804-37-4
323 suppliers
$5.00/250mg
![Methyl 3-[(methoxycarbonylmethyl)sulfamoyl]thiophene-2-carboxylate](/CAS/GIF/106820-63-7.gif)
106820-63-7
150 suppliers
$12.72/1gm:

59804-37-4
323 suppliers
$5.00/250mg
59337-89-2
206 suppliers
$5.00/250mg

59804-37-4
323 suppliers
$5.00/250mg