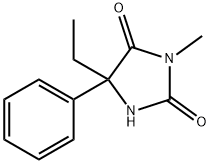
(S)-MEPHENYTOIN
- CAS No.
- 50-12-4
- Chemical Name:
- (S)-MEPHENYTOIN
- Synonyms
- Epilan;Epiazin;Methoin;Metydan;Sacerno;Insulton;Fenantoin;Mesantoin;Mesontoin;NSC-34652
- CBNumber:
- CB0225135
- Molecular Formula:
- C12H14N2O2
- Molecular Weight:
- 218.25
- MOL File:
- 50-12-4.mol
- MSDS File:
- SDS
- Modify Date:
- 2023/6/27 14:18:49
| Melting point | 135-138 °C |
|---|---|
| Boiling point | 358.94°C (rough estimate) |
| Density | 1.154±0.06 g/cm3 (20 ºC 760 Torr) |
| refractive index | 1.6660 (estimate) |
| storage temp. | 2-8°C |
| solubility | DMSO (Slightly), Ethanol (Slightly), Methanol (Slightly) |
| form | solid |
| pka | pKa 8.1 (Uncertain) |
| color | off-white |
| CAS DataBase Reference | 50-12-4(CAS DataBase Reference) |
| EPA Substance Registry System | Mephenytoin (50-12-4) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H302-H315-H319-H335 |
| Precautionary statements | P261-P305+P351+P338 |
| Hazard Codes | Xn |
| Risk Statements | 22-36/37/38 |
| Safety Statements | 26-36 |
| WGK Germany | 2 |
| HS Code | 2933210000 |
| Toxicity | LD50 i.p. in mice: 300 mg/kg (Dzhagatspanyan) |
(S)-MEPHENYTOIN Chemical Properties,Uses,Production
Description
Mephenytoin is N-methylated at position 3 with an ethyl group replacing one of the phenyl substituents at position 5. It is indicated for focal and jacksonian seizures in patients refractory to less toxic AEDs. Mephenytoin produces more sedation than phenytoin and should be used only when safer drugs have failed, because it is associated with an increased incidence of serious toxicities, such as severe rash, agranulocytosis, and hepatitis. Its N-desmethyl metabolite, 5-phenyl- 5-ethylhydantoin, contributes to both efficacy and toxicity for mephenytoin. The drug is no longer commercially available inside but is still available outside the United States.
Uses
Anticonvulsant.
Biological Activity
CYP2C19 substrate. Anticonvulsant.
Side effects
Side effects, including rash, fever, and fatal blood dyscrasia, prevent the use as an anticonvulsant drug of first choice.
Safety Profile
Poison by ingestion and intraperitoneal routes. Human systemic effects by ingestion: somnolence, hemorrhage, changes in teeth and supporting structures. Human mutation data reported. An experimental teratogen. An FDA proprietary drug used as an anticonvulsant. When heated to decomposition it emits toxic fumes of NOx.
(S)-MEPHENYTOIN Preparation Products And Raw materials
Raw materials
1of3
chevron_rightPreparation Products
(S)-MEPHENYTOIN Suppliers
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6351 | 58 | Inquiry |
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | United States | 19892 | 58 | Inquiry |
| InvivoChem | +1-708-310-1919 +1-13798911105 | United States | 6393 | 58 | Inquiry |
| Nantong HI-FUTURE Biology Co., Ltd. | +undefined18051384581 | China | 3136 | 58 | Inquiry |
| Zibo Hangyu Biotechnology Development Co., Ltd | +86-0533-2185556 +8617865335152 | China | 10991 | 58 | Inquiry |
| Shandong Mopai Biotechnology Co., Ltd | 13070690626 13053322091 | China | 11056 | 58 | Inquiry |
| Hubei De'ao Chemical Research Pharmaceutical Technology Co., Ltd | 18502726233; 18502726233 | China | 9808 | 58 | Inquiry |
| BOC Sciences | USA | 0 | 65 | Inquiry | |
| Hefei Mokai Pharmaceutical Technology Co., Ltd | 18130062233 | China | 8000 | 58 | Inquiry |
| Shanghai Maclean Biochemical Technology Co., LTD | 021-50706066 15221275939 | China | 29802 | 58 | Inquiry |
50-12-4((S)-MEPHENYTOIN)Related Search:
1of4
chevron_right




