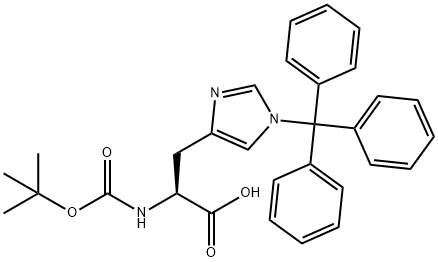
Boc-His(Trt)-OH
- CAS No.
- 32926-43-5
- Chemical Name:
- Boc-His(Trt)-OH
- Synonyms
- BOC-HIS(TRT)-OH;Boc-His(trt);His(Trt)-OH;BOC-L-HIS(TRT)-OH;Boc-L-His(Trt);N-Boc-His(Trt)-OH;(S)-L-Boc-his(trt)-oh;Boc-His(Trt)-OH, >=98%;BOC-N-IM-TRITYL-L-HISTIDINE;N-Boc-N'-trityl-L-histidine
- CBNumber:
- CB0342634
- Molecular Formula:
- C30H31N3O4
- Molecular Weight:
- 497.58
- MOL File:
- 32926-43-5.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/4/23 19:22:53
| Melting point | ~130 °C (dec.) |
|---|---|
| alpha | 12.5 º (C=1% IN MEOH) |
| Boiling point | 667.5±55.0 °C(Predicted) |
| Density | 1.16±0.1 g/cm3(Predicted) |
| storage temp. | Keep in dark place,Sealed in dry,Room Temperature |
| solubility | DMSO (Slightly), Methanol (Sparingly) |
| form | Solid |
| pka | 3.17±0.10(Predicted) |
| color | White to Off-White |
| optical activity | [α]20/D +12.5±1.0°, c = 1% in methanol |
| BRN | 732035 |
| InChIKey | OYXZPXVCRAAKCM-SANMLTNESA-N |
| SMILES | C(O)(=O)[C@H](CC1N=CN(C(C2=CC=CC=C2)(C2=CC=CC=C2)C2=CC=CC=C2)C=1)NC(OC(C)(C)C)=O |
| CAS DataBase Reference | 32926-43-5(CAS DataBase Reference) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H315-H319 |
| Precautionary statements | P264-P280-P302+P352-P337+P313-P305+P351+P338-P362+P364-P332+P313 |
| WGK Germany | 3 |
| HS Code | 29224999 |
Boc-His(Trt)-OH price More Price(3)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | 8.53034 | Boc-His(Trt)-OH Novabiochem? | 32926-43-5 | 25G | ₹96130.01 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 8.53034 | Boc-His(Trt)-OH Novabiochem? | 32926-43-5 | 8530340100 | ₹292540 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 15449 | Boc-His(Trt)-OH ≥98.0% (TLC) | 32926-43-5 | 1G | ₹9298.68 | 2022-06-14 | Buy |
Boc-His(Trt)-OH Chemical Properties,Uses,Production
Chemical Properties
White to off white powder
Uses
Boc-His(Trt)-OH was used in the study to prepare and study structure-activity relationship of amino acid amides as selective inhibitors for dipeptidyl peptidases.
Uses
Boc-His(Trt)-OH is a histidine derivative. Its a protected histidine derivative for only the critical coupling step. After coupling the Boc- and Trt-groups are cleaved simultaneously by TFA.
Preparation
The benzyl 2-tert-butoxycarbonylamino-3-(1-trimethylimidazol-4-yl)propionate was dissolved in methanol (20 mL), and potassium hydroxide was added to the reaction system. The reaction system was stirred for 1 h after THF was added to the reaction system. The solvent is removed under vacuum depressurization, and the mixture is then extracted with water and Et2O. Separate the two phases and acidify the aqueous layer with dilute HCl, extract it with Et2O, combine all the ether organic layer and wash it with water and saline, dry the organic layer with anhydrous magnesium sulfate, filter to remove the solvent, and concentrate the filtrate under vacuum under reduced pressure to obtain Boc-His(Trt)-OH.
Application
N-Boc-N'-Trityl-L-histidine (Boc-His(Trt)-OH) can be used as an intermediate in biochemistry and medicinal chemistry and can be used in the synthesis of peptide drug molecules, vitamins, and molecules with specific biologically active functions. In organic synthesis transformation, the carboxyl group in N-Boc-N'-trityl-L-histidine can undergo corresponding condensation reactions with amine compounds to obtain the corresponding amide products; in addition, in dithionyl chloride Under the action of N-Boc-N'-trityl-L-histidine, intramolecular dehydration cyclization reaction can be performed to obtain cyclic amide derivatives.
reaction suitability
Reaction type: Boc solid-phase peptide synthesis
Boc-His(Trt)-OH Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| JSK Chemicals | +919879767970 | Gujarat, India | 3756 | 58 | Inquiry |
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6351 | 58 | Inquiry |
| A.J Chemicals | 91-9810153283 | New Delhi, India | 6124 | 58 | Inquiry |
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 | China | 20361 | 58 | Inquiry |
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 | China | 29798 | 60 | Inquiry |
| Shanghai Daken Advanced Materials Co.,Ltd | +86-371-66670886 | China | 16220 | 58 | Inquiry |
| Hangzhou FandaChem Co.,Ltd. | 008657128800458; +8615858145714 | China | 9338 | 55 | Inquiry |
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 | China | 32760 | 60 | Inquiry |
| career henan chemical co | +86-0371-86658258 +8613203830695 | China | 29901 | 58 | Inquiry |
| Xiamen AmoyChem Co., Ltd | +86-592-6051114 +8618959220845 | China | 6387 | 58 | Inquiry |





