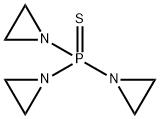
Triethylenethiophosphoramide
- CAS No.
- 52-24-4
- Chemical Name:
- Triethylenethiophosphoramide
- Synonyms
- stepa;tespa;sk6882;tifosyl;nsc6396;THIOTEF;TIO-TEF;Sertepa;hiotepa;wr-45312
- CBNumber:
- CB0458870
- Molecular Formula:
- C6H12N3PS
- Molecular Weight:
- 189.22
- MOL File:
- 52-24-4.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/7/12 17:58:41
| Melting point | 54-57 °C |
|---|---|
| Boiling point | 270.2±23.0 °C(Predicted) |
| Density | 1.50±0.1 g/cm3(Predicted) |
| storage temp. | 2-8°C |
| solubility | Soluble in benzene, acetone and methanol. |
| pka | 2.74±0.20(Predicted) |
| form | solid |
| color | white |
| Water Solubility | 19 g/100 mL (25 ºC) |
| Stability | Stable. Incompatible with strong oxidizing agents. |
| InChIKey | FOCVUCIESVLUNU-UHFFFAOYSA-N |
| CAS DataBase Reference | 52-24-4 |
| IARC | 1 (Vol. Sup 7, 50, 100A) 2012 |
| NIST Chemistry Reference | Thiotepa(52-24-4) |
| EPA Substance Registry System | Thiotepa (52-24-4) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS06,GHS08 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H300-H350 | |||||||||
| Precautionary statements | P201-P280-P301+P330+P331+P310-P308+P313 | |||||||||
| Hazard Codes | T+ | |||||||||
| Risk Statements | 45-46-28 | |||||||||
| Safety Statements | 53-22-26-36/37/39-45-36/37-28 | |||||||||
| RIDADR | UN 2811 6.1/PG 2 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | SZ2975000 | |||||||||
| HazardClass | 6.1(a) | |||||||||
| PackingGroup | II | |||||||||
| HS Code | 2933999552 | |||||||||
| Toxicity | LD50 i.v. in rats: 15 mg/kg (Scherf) | |||||||||
| NFPA 704 |
|
Triethylenethiophosphoramide Chemical Properties,Uses,Production
Description
Thiotepa, a tertiary aziridine, is less reactive than quaternary aziridinium compounds and is classified as a weak alkylator. It is possible for the nitrogen atoms to be protonate before reacting with DNA (a positively charged aziridine is more reactive than the un-ionized aziridine), but the electron-withdrawing effect of the sulfur atom decreases the pKa to approximately six, which keeps the percentage ionized at pH 7.4 relatively low. Thiotepa undergoes oxidative desulfuration, forming an active cytotoxic metabolite known as TEPA (triethylenephosphoramide).
Chemical Properties
white crystals or powder
Uses
Tri(1-aziridinyl)phosphine sulfide is useful for the treatment of cancers, especially cancers resistant to chemotherapy. Antineoplastic. Thio-TEPA (N,N?N?-triethylenethiophosphoramide) is used as a cancer chemotherapeutic, alkylating agent. It is used to treat various kinds of cancer such as breast, ovarian and bladder cancer. It is also used as conditioning treatment prior to hematopoietic progenitor cell transplantation (HPCT)
Indications
Although thiotepa is chemically less reactive than the nitrogen
mustards, it is thought to act by similar mechanisms.
Its oral absorption is erratic. After intravenous injection,
the plasma half-life is less than 2 hours. Urinary
excretion accounts for 60 to 80% of eliminated drug.
Thiotepa has antitumor activity against ovarian and
breast cancers and lymphomas. However, it has been
largely supplanted by cyclophosphamide and other nitrogen
mustards for treatment of these diseases. It is
used by direct instillation into the bladder for multifocal
local bladder carcinoma.
Nausea and myelosuppression are the major toxicities
of thiotepa. It is not a local vesicant and has been
safely injected intramuscularly and even intrathecally.
General Description
Odorless white crystalline solid.
Air & Water Reactions
Water soluble.
Reactivity Profile
Triethylenethiophosphoramide polymerizes readily upon exposure to heat or moisture, especially at acidic pH.
Hazard
Confirmed carcinogen.
Fire Hazard
Flash point data for Triethylenethiophosphoramide are not available. Triethylenethiophosphoramide is probably combustible.
Mechanism of action
Thiotepa and the TEPA metabolite readily enter the CNS after systemic administration, leading to dizziness, blurred vision, and headaches. More critically, these agents also are severe myelosuppressants and can induce leukopenia, thrombocytopenia, and anemia. Patients treated with thiotepa are at high risk for infection and hemorrhage.
Clinical Use
This antineoplastic agent is most commonly employed in the treatment of ovarian and breast cancers, as well as papillary carcinoma of the bladder.
Side effects
Patients have died from myelosuppression after intravesically administered thiotepa. The drug also causes damage to the hepatic and renal systems. Dose and/or administration frequency should be increased slowly, even if the initial response to the drug is sluggish, or unacceptable toxicity may result.
Safety Profile
Confirmed human carcinogen producing leukemia. Poison by ingestion, intraperitoneal, intravenous, and subcutaneous routes. Experimental teratogenic data. Human systemic effects by parenteral route: paresthesia, bone marrow changes, and leukemia. Experimental reproductive effects. Human mutation data reported. When heated to decomposition it emits very toxic fumes of POx, SOx, and NOx.
Potential Exposure
Used in the treatment of cancers resistant to chemotherapy. Antineoplastic: thiotepa has been prescribed for a wide variety of neoplastic diseases: adenocarcinomas of the breast and the ovary; superficial carcinoma of the urinary bladder; controlling intracavitary or localized neoplastic disease; lymphomas, such aslymphosarcomas and Hodgkin’s disease; as well as bronchogenic carcinoma.
Carcinogenicity
Thiotepa is known to be a human carcinogen based on sufficient evidence from studies in humans. Thiotepa was first listed in the Second Annual Report on Carcinogens in 1981 as reasonably anticipated to be a human carcinogen based on sufficient evidence of carcinogenicity from studies in experimental animals and insufficient evidenceof carcinogenicity from studies in humans. Thiotepa was reclassified as known to be a human carcinogen in the Eighth Report on Carcinogens in 1998.
Shipping
UN2811 Toxic solids, organic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required. UN3249 Medicine, solid, toxic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials.
Incompatibilities
Tris(aziridinyl)phosphine sulfide polymerizes readily upon exposure to heat or moisture, especially at acidic pH. Incompatible with strong oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides.
Waste Disposal
It is inappropriate and possibly dangerous to the environment to dispose of expired or waste pharmaceuticals by flushing them down the toilet or discarding them to the trash. Household quantities of expired or waste pharmaceuticals may be mixed with wet cat litter or coffee grounds, double-bagged in plastic, discard in trash. Larger quantities shall carefully take into consideration applicable DEA, EPA, and FDA regulations. If possible return the pharmaceutical to the manufacturer for proper disposal being careful to properly label and securely package the material. Alternatively, the waste pharmaceutical shall be labeled, securely packaged and transported by a state licensed medical waste contractor to dispose by burial in a licensed hazardous or toxic waste landfill or incinerator.
Triethylenethiophosphoramide Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Basil Drugs AND Pharmaceuticals Pvt Ltd | +91-2249700250 +91-9619320820 | Mumbai, India | 108 | 58 | Inquiry |
| NATCO Pharma Limited | +91-9849511345 +91-9866005199 | Hyderabad, India | 24 | 58 | Inquiry |
| Mylan Laboratories Ltd | +91-4023550543 +91-4030866666 | Telangana, India | 150 | 58 | Inquiry |
| Aspen Biopharma Labs Pvt Ltd | +91-9248058660 +91-9248058662 | Telangana, India | 234 | 58 | Inquiry |
| MSN LABORATORIES PRIVATE LTD | +91 40 30438600 | New Delhi, India | 230 | 58 | Inquiry |
| EMCURE PHARMACEUTICALS LTD | +91 22 3061 0000 | New Delhi, India | 93 | 58 | Inquiry |
| Pharmaffiliates Analytics and Synthetics P. Ltd | +91-172-5066494 | Haryana, India | 6773 | 58 | Inquiry |
| SynZeal Research Pvt Ltd | +1 226-802-2078 | Gujarat, India | 6522 | 58 | Inquiry |
| Shri Swami Samarth Surgicals | 07942548874 | Maharashtra, India | 5 | 58 | Inquiry |
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6351 | 58 | Inquiry |
52-24-4(Triethylenethiophosphoramide)Related Search:
1of4
chevron_right




