K 02288
- CAS No.
- 1431985-92-0
- Chemical Name:
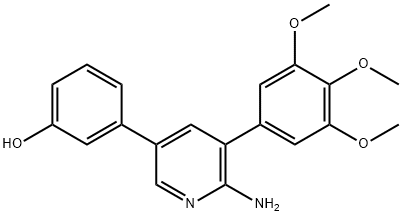
- K 02288
- Synonyms
- CS-1877;K 02288;K 02288a;LM-3151 K02288;K 02288(K 02288a);K 02288 USP/EP/BP;K02288;K 02288;K-02288;3-[6-Amino-5-(3,4,5-trimethoxyphenyl)pyridin-3-yl]phenol;3-[(6-Amino-5-(3,4,5-trimethoxyphenyl)-3-pyridinyl]phenol;Phenol, 3-[6-amino-5-(3,4,5-trimethoxyphenyl)-3-pyridinyl]-
- CBNumber:
- CB12677546
- Molecular Formula:
- C20H20N2O4
- Molecular Weight:
- 352.38
- MOL File:
- 1431985-92-0.mol
- MSDS File:
- SDS
- Modify Date:
- 2023/6/30 15:45:59
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS05,GHS06 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H301-H318 | |||||||||
| Precautionary statements | P280-P301+P310+P330-P305+P351+P338+P310 | |||||||||
| RIDADR | UN 2811 6.1 / PGIII | |||||||||
| HS Code | 2933.39.9200 | |||||||||
| NFPA 704 |
|
K 02288 Chemical Properties,Uses,Production
Description
Activation of bone morphogenetic protein (BMP) type I receptors, also known as activin receptor-like kinases (ALK1-7), leads to the assembly of SMAD complexes, which translocate to the nucleus to induce transcriptional activation important for normal development and tissue repair. K02288 is a 2-aminopyridine-based inhibitor of ALK1 and ALK2 with IC50 values of 1.8 and 1.1 nM, respectively. It is less selective for ALK3, 4, 5, and 6 subtypes and the type II BMP receptor ActRIIA, demonstrating IC50 values of 34.4, 302, 321, 6.4, and 220 nM, respectively. K02288 can prevent BMP4-induced SMAD1/5/8 pathway activation in vitro (IC50 = 100 nM) without affecting TGF-β signaling. Furthermore, at 8-10 μM, K02288 has been used to induce the dorsalization of zebrafish embryos. Through specific inhibition of BMP signaling, this compound can be used to research stem cell biology and disease models of musculoskeletal dysplasia and cancer.
Uses
K02288 is a potent and selective type 1 BMP (bone morphogenetic protein) receptor inhibitor with in vitro activity against ALK-2 (activin receptor-like kinase-2) at low nanomolar concentrations. K02288 also inhibits the BMP-induced Smad pathway without affecting TGF-β signaling and induces dorsalization of zebrafish embryos.
General Description
K02288 is a 2-aminopyridine compound.
K 02288 Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6351 | 58 | Inquiry |
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 | China | 32760 | 60 | Inquiry |
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | United States | 19892 | 58 | Inquiry |
| Dideu Industries Group Limited | +86-29-89586680 +86-15129568250 | China | 25247 | 58 | Inquiry |
| InvivoChem | +1-708-310-1919 +1-13798911105 | United States | 6393 | 58 | Inquiry |
| Coresyn Pharmatech Co., Ltd. | +86-571-86626709 +86-18157142896 | China | 9991 | 58 | Inquiry |
| ShenZhen Trendseen Biological Technology Co.,Ltd. | 13417589054 | China | 11681 | 58 | Inquiry |
| Wuhan Topule Biopharmaceutical Co., Ltd | +8618327326525 | China | 8474 | 58 | Inquiry |
| Shenyang Zhongshen Zekang Biomedical Technology Research Co., Ltd | +86-18341751992 +86-15382112998 | China | 892 | 58 | Inquiry |
| Aladdin Scientific | +1-+1(833)-552-7181 | United States | 57511 | 58 | Inquiry |
1431985-92-0(K 02288)Related Search:
1of2
chevron_right




