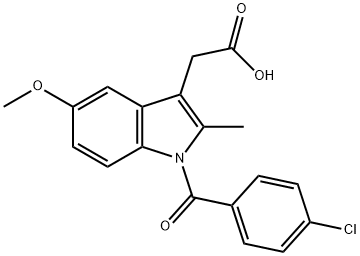
Indometacin
- CAS No.
- 53-86-1
- Chemical Name:
- Indometacin
- Synonyms
- 53-86-1;INDOMETHACIN;INDOMETHACINE;2-(1-(4-chlorobenzoyl)-5-Methoxy-2-Methyl-1H-indol-3-yl)acetic acid;INDOCIN;ndomethacin;Catlep;Inteban;ndometacin;INDOMETACINE
- CBNumber:
- CB1750267
- Molecular Formula:
- C19H16ClNO4
- Molecular Weight:
- 357.79
- MOL File:
- 53-86-1.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/8/15 19:00:21
| Melting point | 158-162 °C |
|---|---|
| Boiling point | 499.4±45.0 °C(Predicted) |
| Density | 1.2135 (rough estimate) |
| refractive index | 1.6800 (estimate) |
| storage temp. | Store at RT |
| solubility | ethanol: 50 mg/mL, clear, yellow-green |
| form | White to off-white powder |
| pka | 4.5(at 25℃) |
| color | White to Light yellow to Light orange |
| Water Solubility | Soluble in acetone (40 mg/mL - clear, yellow solution), ethanol (20 mg/mL), ether, castor oil; Soluble in chloroform (50 mg/mL - clear, yellow, extremely viscous solution); decomposed by strong alkali but stable in neutral or slightly acidic media; insoluble in water. |
| Sensitive | Light Sensitive |
| Merck | 14,4968 |
| BRN | 497341 |
| BCS Class | 2 |
| Stability | Stable. Incompatible with strong oxidizing agents. |
| InChIKey | CGIGDMFJXJATDK-UHFFFAOYSA-N |
| CAS DataBase Reference | 53-86-1(CAS DataBase Reference) |
| NIST Chemistry Reference | Indomethacin(53-86-1) |
| EPA Substance Registry System | Indomethacin (53-86-1) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS06 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H300 | |||||||||
| Precautionary statements | P264-P301+P310 | |||||||||
| Hazard Codes | T+,Xi,T | |||||||||
| Risk Statements | 28-36/37/38-39/23/24/25-23/24/25 | |||||||||
| Safety Statements | 28-36/37-45-36-26 | |||||||||
| RIDADR | UN 2811 6.1/PG 1 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | NL3500000 | |||||||||
| F | 8-10 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 6.1 | |||||||||
| PackingGroup | I | |||||||||
| HS Code | 29339900 | |||||||||
| Toxicity | LD50 i.p. in rats: 13 mg/kg (Klaassen) | |||||||||
| NFPA 704 |
|
Indometacin price More Price(12)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | I8280 | Indomethacin meets USP testing specifications | 53-86-1 | 5G | ₹3399.05 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | PHR1247 | Indomethacin Pharmaceutical Secondary Standard; Certified Reference Material | 53-86-1 | 500MG | ₹10695.1 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | I8280 | Indomethacin meets USP testing specifications | 53-86-1 | 25G | ₹13596.2 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | I7378 | Indomethacin 98.5-100.5% (in accordance with EP) | 53-86-1 | 5G | ₹8151.23 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | I8280 | Indomethacin meets USP testing specifications | 53-86-1 | 100G | ₹42444.83 | 2022-06-14 | Buy |
Indometacin Chemical Properties,Uses,Production
Description
Aqueous solutions of indomethacin are not stable because of the ease of hydrolysis of the p-chlorobenzoyl group. The original synthesis of indomethacin by Shen et al. involved the formation of 2-methyl-5-methoxyindole acetic acid and subsequent acylation after protection of the carboxyl group as the t-butyl ester. It was introduced in the United States in 1965. It is still one of the most potent NSAIDs in use. It also is a more potent antipyretic than either aspirin or acetaminophen, and it possesses approximately 10 times the analgetic potency of aspirin.
Chemical Properties
Crystalline Solid
Uses
Inhibits cyclooxygenase (IC50=0.1uM) selectively over liposygenases (IC50=100uM for 5-,12- and 15-LO). A clinically useful NAISD
Indications
Indomethacin (Indocin) is used in the treatment of acute gouty arthritis, rheumatoid arthritis, ankylosing spondylitis, and osteoarthritis. It is not recommended for use as a simple analgesic or antipyretic because of its potential for toxicity.While indomethacin inhibits both COX-1 and COX-2, it is moderately selective for COX- 1. It produces more CNS side effects than most of the other NSAIDs. Severe headache occurs in 25 to 50% of patients; vertigo, confusion, and psychological disturbances occur with some regularity. GI symptoms also are more frequent and severe than with most other NSAIDs. Hematopoietic side effects (e.g., leukopenia, hemolytic anemia, aplastic anemia, purpura, thrombocytopenia, and agranulocytosis) also may occur. Ocular effects (blurred vision, corneal deposits) have been observed in patients receiving indomethacin, and regular ophthalmological examinations are necessary when the drug is used for long periods. Hepatitis, jaundice, pancreatitis, and hypersensitivity reactions also have been noted.
Definition
The antiinflammatory drug indomethacin.
Preparation
acylation of sodium 2-(4-
methoxyphenyl)hydrazine-1-sulfonate with
4-chlorobenzoyl chloride followed by
heating yields 1-(4- chlorobenzoyl)-1-(4-
methoxyphenyl)hydrazine. Condensation with
levulinic acid in a Fischer indole synthesis affords indomethacin.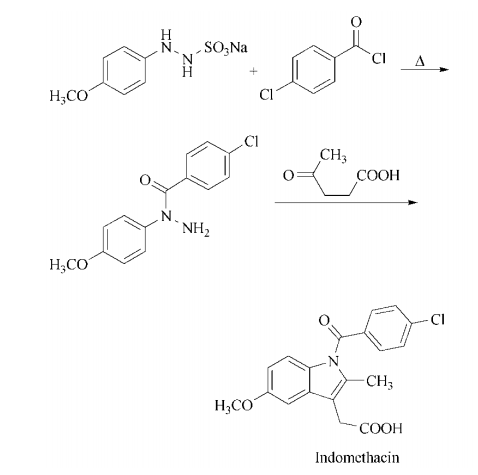
brand name
Amuno (MSD Sharp & Dohme, Germany), Arthrexin (Lennon Generics, South Africa), Confortid (Dumex, Denmark, Sweden, Finland), Doctucid (Coctum, Greece), Dynamectin (Dynamed, South Africa), Flexidin (Mundipharma, Austria), Inacid (Merck Sharp & Dohme, Spain), Indobene (Merckle, Austria, CIS), Indocin (Merck, USA), Reumadolor (Bros, Greece), Zoflam (Norpharma, Denmark).
World Health Organization (WHO)
Indometacin was introduced in 1963 and it is one of the first NSAIDs. Convulsions are rarely reported in relation with the use of this group of agents. Indometacin farnesil is a pro-drug of indometacin, and the occurrence of gastro-intestinal adverse effects could be expected. See also under nonsteroidal antiinflammatory agents.
Biological Functions
Indomethacin (Indocin) is an acetic acid derivative related functionally to sulindac (Clinoril), a prodrug with a long half-life, and etodolac (Lodine).They are metabolized in the liver and excreted as metabolites in the bile and via the kidney. They are potent inhibitors of COX and thus extremely effective antiinflammatory agents.
General Description
Crystals.
Air & Water Reactions
Practically insoluble in water. Decomposes in alkali.
Reactivity Profile
A weak organic acid.
Fire Hazard
Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Some are oxidizers and may ignite combustibles (wood, paper, oil, clothing, etc.). Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated.
Pharmaceutical Applications
Indomethacin is a nonsteroidal anti-inflammatory agent used in pain and moderate to severe
inflammation in rheumatic diseases and other musculoskeletal disorders. It is a COX (cyclooxygenase)
inhibitor and therefore interrupts the production of prostaglandins.
A series of new silicon compounds, based on the structure of indomethacin, have been synthesised and
are under investigation as novel anticancer agents. The carboxyl group of indomethacin was reacted with
a series of amino-functionalised silanes. The resulting products have been shown to be significantly more
lipophilic and more selective to COX-2. Furthermore, in vitro testing has shown an increased uptake of the
new compounds at the tumour site. The silane-functionalised indomethacin derivatives exhibited a 15-fold
increased antiproliferative effect when tested against pancreatic cancer .
Biological Activity
Cyclooxgenase (COX) inhibitor; displays selectivity for COX-1 (IC 50 values are 230 and 630 nM for human COX-1 and COX-2 respectively). Widely used anti-inflammatory agent.
Clinical Use
Indomethacin is available for the short-term treatment of acute gouty arthritis, acute pain of ankylosing spondylitis, and osteoarthritis. An injectable form to be reconstituted also is available as the sodium trihydrate salt for IV use in premature infants with patent ductus arteriosus. Because of its ability to suppress uterine activity by inhibiting prostaglandin biosynthesis, indomethacin also has an unlabeled use to prevent premature labor.
Side effects
All of these drugs produce analgesic effects, antipyresis, and antiinflammatory effects.Due to the high incidence of gastric irritation, headache, nausea, and other side effects, including hematological effects and coronary vasoconstriction, they are not useful as an initial treatment for pain. GI irritation and ulceration occur to a lesser extent with etodolac. Indomethacin is useful in the treatment of acute gout, osteoarthritis, ankylosing spondylitis, and acceleration of the closure of the ductus arteriosus in premature infants. The tocolytic effects of indomethacin to prevent preterm labor are the result of its effects on prostaglandin synthesis. However, the toxicity of the drug limits such application, since it increases fetal morbidity. Indomethacin is contraindicated in pregnancy, in asthmatics, and in those with gastric ulcers or other ulceration of the GI tract. Indomethacin may increase the symptoms associated with depression or other psychiatric disturbances and those associated with epilepsy and Parkinson’s disease. The drug should be used with caution in such patients.
Indometacin Preparation Products And Raw materials
Raw materials
1of2
chevron_rightPreparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Jigs Chemical ltd | +919099003427 | Gujarat, India | 239 | 58 | Inquiry |
| OPULENT PHARMA | +91-9106692785 +91-9106692785 | Gujarat, India | 479 | 58 | Inquiry |
| ANWITA APIS | +919000311012 | Hyderabad, India | 198 | 58 | Inquiry |
| Global Calcium Pvt Ltd | +91-8050750904 +91-8040554500 | Karnataka, India | 132 | 58 | Inquiry |
| Macleods Pharmaceuticals Limited | +91-2266762800 +91-2266762800 | Maharashtra, India | 116 | 58 | Inquiry |
| Horster Biotek Pvt Ltd | +91-7359657360 +91-9898127219 | Gujarat, India | 58 | 58 | Inquiry |
| Medi Pharma Drug House | +919930911911 | Mumbai, India | 143 | 58 | Inquiry |
| AKASH PHARMA EXPORTS | +91-9388123451 +91-9846039283 | Kerela, India | 470 | 58 | Inquiry |
| HETERO DRUGS LTD | +91 40 23704923 / 24 / 25 | New Delhi, India | 98 | 58 | Inquiry |
| Medi Pharma Drug House | 91-22-61904500 (100 Lines) | Maharashtra, India | 81 | 58 | Inquiry |
| Supplier | Advantage |
|---|---|
| Jigs Chemical ltd | 58 |
| OPULENT PHARMA | 58 |
| ANWITA APIS | 58 |
| Global Calcium Pvt Ltd | 58 |
| Macleods Pharmaceuticals Limited | 58 |
| Horster Biotek Pvt Ltd | 58 |
| Medi Pharma Drug House | 58 |
| AKASH PHARMA EXPORTS | 58 |
| HETERO DRUGS LTD | 58 |
| Medi Pharma Drug House | 58 |
Related articles
- Indomethacin: Pharmacokinetics and Mechanism of Action
- Indomethacin displays rapid absorption, dose-proportional peak plasma concentrations and unique actions in conditions like tri....
- May 29,2024
- Pharmacological effects of indomethacin
- Indomethacin is suitable for antipyretic and inflammatory pain relief
- Mar 28,2022





