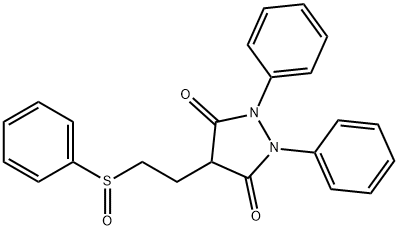
(+/-)-Sulfinpyrazone
- CAS No.
- 57-96-5
- Chemical Name:
- (+/-)-Sulfinpyrazone
- Synonyms
- g28315;anturan;anturen;enturan;enturen;g28,315;anturane;anturano;anturanil;anturidin
- CBNumber:
- CB2122351
- Molecular Formula:
- C23H20N2O3S
- Molecular Weight:
- 404.48
- MOL File:
- 57-96-5.mol
- Modify Date:
- 2024/7/2 8:55:02
| Melting point | 136-137° |
|---|---|
| Boiling point | 590.8±42.0 °C(Predicted) |
| Density | 1.1890 (rough estimate) |
| refractive index | 1.6360 (estimate) |
| storage temp. | Sealed in dry,Room Temperature |
| solubility | Very slightly soluble in water, sparingly soluble in ethanol (96 per cent). It dissolves in dilute solutions of alkali hydroxides. |
| form | Solid |
| pka | pKa 2.8(H2O t = RT I undefined) (Uncertain) |
| color | Off-White to Light Brown |
| Water Solubility | 2.601g/L(22 ºC) |
| CAS DataBase Reference | 57-96-5(CAS DataBase Reference) |
| NIST Chemistry Reference | Sulfinpyrazone(57-96-5) |
(+/-)-Sulfinpyrazone price More Price(3)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | S9509 | (±)-Sulfinpyrazone analytical standard | 57-96-5 | 5G | ₹6798.1 | 2022-06-14 | Buy |
| TCI Chemicals (India) | U0114 | Sulfinpyrazone | 57-96-5 | 5G | ₹3300 | 2022-05-26 | Buy |
| TCI Chemicals (India) | U0114 | Sulfinpyrazone | 57-96-5 | 25G | ₹9900 | 2022-05-26 | Buy |
(+/-)-Sulfinpyrazone Chemical Properties,Uses,Production
Description
Sulfinpyrazone is soluble in alkaline solutions. Its synthesis is similar to that of phenylbutazone (Fig. 36.33). It produces its uricosuric effect in a manner similar to that of probenecid. A dose of 35 mg produces a uricosuric effect equivalent to that produced by 100 mg of probenecid, whereas 400 mg/day of sulfinpyrazone produces an effect comparable to that obtained with doses of 1.5 to 2 g of probenecid. It also possesses, not surprisingly, some of the properties of phenylbutazone. It is an inhibitor of human platelet prostaglandin synthesis at the cyclooxygenase step, resulting in a decrease in platelet release and a reduction in platelet aggregation. This antiplatelet effect suggests a role for sulfinpyrazone in reducing the incidence of sudden death, which can occur in the first year following a myocardial infarction; however, it lacks the analgetic and anti-inflammatory effects of phenylbutazone.
Chemical Properties
White or almost white powder.
Uses
As an anti-inflammatory and uricosuric agent, its side effects were severe enough to preclude its continuous use in the treatment of chronic gout. Evaluation of several chemical congeners indicated that the phenylthioethyl analog of phenylbutazone had promising anti-inflammatory and uricosuric activity. A metabolite, the sulfoxide pyrazone, exhibited enhanced uricosuric activity. Interestingly, the corresponding sulfone does not appear to be a metabolite. Sulfinpyrazone lacks the clinically striking anti-inflammatory and analgesic properties of phenylbutazone.
Indications
Sulfinpyrazone (Anturane), another uricosuric agent, is chemically related to the antiinflammatory and uricosuric compound phenylbutazone. However, it lacks the antiinflammatory, analgesic, and sodium-retaining properties of phenylbutazone and possesses a number of undesirable side effects that limit its therapeutic usefulness.
General Description
Sulfinpyrazone (Anturane) produces its uricosuric action ina similar manner to that of probenecid and is indicated forthe treatment of chronic and recurrent gouty arthritis. It iswell absorbed with approximately 50% of the administereddose excreted as unchanged drug into the renal tubules. Therest of the drug is primarily metabolized via CYP2C9 intothe corresponding sulfide and sulfone metabolites, thus itcan potentiate the anticoagulant effect of warfarin.
Mechanism of action
The mechanism of sulfinpyrazone’s uricosuric activity is similar to that of probenecid. Sulfinpyrazone is readily absorbed after oral administration, with peak blood levels reached 1 to 2 hours after ingestion. It is more highly bound to plasma protein (98–99%) than is probenecid (84–94%) and is a more potent uricosuric agent. Most of the drug (90%) is eliminated through active proximal tubular secretion of the intact parent compound. Sulfinpyrazone also undergoes p-hydroxylation to form a uricosuric metabolite, the formation of which undoubtedly contributes to the drug’s prolonged activity (about 10 hours) and potency relative to probenecid. In contrast to probenecid, the rate of excretion of sulfinpyrazone is not enhanced by alkalinization of the urine, since the drug is largely ionized at all urinary pH ranges and therefore not a candidate for passive back-diffusion.
Pharmacokinetics
Only the parent sulfinpyrazone and its reduced sulfide metabolite, however, are active as COX inhibitors. Because these compounds are reversible inhibitors, the antithrombotic activity lasts only as long as blood levels of the drug and metabolite persist (half-life, 4–6 hours for parent sulfinpyrazone, 11–14 hours for the sulfide metabolite). Sulfinpyrazone is not yet approved in the United States for use in acute myocardial infarction or for transient ischemic attack prophylaxis.
Pharmacology
Sulfinpyrazone, although less effective than allopurinol in reducing serum uric acid levels, remains useful for the prevention or reduction of the joint changes and tophus deposition that would otherwise occur in chronic gout; it has no antiinflammatory properties. During the initial period of sulfinpyrazone use, acute attacks of gout may increase in frequency and severity. It is recommended, therefore, that either colchicine or a nonsteroidal antiinflammatory agent be coadministered during early sulfinpyrazone therapy. Abdominal pain, nausea, and possible reactivation of peptic ulcer have been reported.
Clinical Use
Sulfinpyrazone is a structural derivative of the anti-inflammatory drug phenylbutazone. Unlike phenylbutazone, however, sulfinpyrazone does not have significant anti-inflammatory activity. It does have potent uricosuric effects and frequently is used in the treatment of gout.
Side effects
The most frequent adverse reactions are GI disturbances; however, the incidence is relatively low.
Metabolism
The metabolites produced result from sulfoxide reduction, sulfur and aromatic oxidation, and C-glucuronidation of the heterocyclic ring in a manner similar to that for phenylbutazone. The metabolite resulting from p-hydroxylation of the aromatic ring possesses uricosuric effects in humans. The sulfide metabolite, a major metabolic product, may contribute to the antiplatelet effects of sulfinpyrazone but not to the uricosuric effects.
(+/-)-Sulfinpyrazone Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| A.J Chemicals | 91-9810153283 | New Delhi, India | 6124 | 58 | Inquiry |
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6351 | 58 | Inquiry |
| TCI Chemicals (India) Pvt. Ltd. | 1800 425 7889 | New Delhi, India | 6778 | 58 | Inquiry |
| Pharmaffiliates Analytics and Synthetics P. Ltd | +91-172-5066494 | Haryana, India | 6773 | 58 | Inquiry |
| career henan chemical co | +86-0371-86658258 +8613203830695 | China | 29897 | 58 | Inquiry |
| Chongqing Chemdad Co., Ltd | +86-023-6139-8061 +86-86-13650506873 | China | 39916 | 58 | Inquiry |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | China | 49391 | 58 | Inquiry |
| Zhengzhou Alfa Chemical Co.,Ltd | +8618530059196 | China | 13102 | 58 | Inquiry |
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 | China | 12458 | 58 | Inquiry |
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | United States | 19892 | 58 | Inquiry |
57-96-5((+/-)-Sulfinpyrazone )Related Search:
1of4
chevron_right





