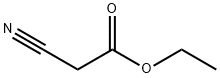
Ethyl cyanoacetate
- CAS No.
- 105-56-6
- Chemical Name:
- Ethyl cyanoacetate
- Synonyms
- ETHYL 2-CYANOACETATE;CYANOACETIC ACID ETHYL ESTER;ECYA;NCCH2COOC2H5;2-Ethenylpyrazine;Ethyl cyanacetate;CL079;usafkf-25;USAF kf-25;Cyanessigester
- CBNumber:
- CB9852660
- Molecular Formula:
- C5H7NO2
- Molecular Weight:
- 113.11
- MOL File:
- 105-56-6.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/3/14 15:18:33
| Melting point | -22 °C (lit.) |
|---|---|
| Boiling point | 208-210 °C (lit.) |
| Density | 1.063 g/mL at 25 °C (lit.) |
| vapor density | 3.9 (vs air) |
| vapor pressure | 1 mm Hg ( 67.8 °C) |
| refractive index |
n |
| Flash point | >230 °F |
| storage temp. | Store below +30°C. |
| solubility | 20g/l |
| form | Liquid |
| pka | 3.19±0.10(Predicted) |
| color | Clear |
| Water Solubility | 20 g/L (20 ºC) |
| Merck | 14,3786 |
| BRN | 605871 |
| Dielectric constant | 26.9(20℃) |
| LogP | -0.119-1.05 at 23-25℃ and pH6.1 |
| Surface tension | 70.2mN/m at 1.034g/L and 23℃ |
| CAS DataBase Reference | 105-56-6(CAS DataBase Reference) |
| NIST Chemistry Reference | Acetic acid, cyano-, ethyl ester(105-56-6) |
| EPA Substance Registry System | Ethyl cyanoacetate (105-56-6) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H319 | |||||||||
| Precautionary statements | P264-P280-P305+P351+P338-P337+P313 | |||||||||
| Hazard Codes | Xn,Xi | |||||||||
| Risk Statements | 20/21/22-36/38 | |||||||||
| Safety Statements | 36/37-37/39-26 | |||||||||
| RIDADR | 3276 | |||||||||
| WGK Germany | 1 | |||||||||
| RTECS | AG4110000 | |||||||||
| F | 10 | |||||||||
| Autoignition Temperature | 460 °C | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 2926 90 70 | |||||||||
| Toxicity | LD50 orally in Rabbit: > 2000 mg/kg | |||||||||
| NFPA 704 |
|
Ethyl cyanoacetate price More Price(11)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | E18425 | Ethyl cyanoacetate ≥98% | 105-56-6 | 5G | ₹1916.03 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | E18425 | Ethyl cyanoacetate ≥98% | 105-56-6 | 250G | ₹2652.13 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | E18425 | Ethyl cyanoacetate ≥98% | 105-56-6 | 1KG | ₹5520.75 | 2022-06-14 | Buy |
| ALFA India | ALF-A11498-36 | Ethyl cyanoacetate, 98+% | 105-56-6 | 500g | ₹4035 | 2022-05-26 | Buy |
| ALFA India | ALF-A11498-0E | Ethyl cyanoacetate, 98+% | 105-56-6 | 2500g | ₹10929 | 2022-05-26 | Buy |
Ethyl cyanoacetate Chemical Properties,Uses,Production
Description
Ethyl cyanoacetate is the ethyl ester of cyanoacetic acid. Ethyl cyanoacetate hydrolizes rapidly under neutral and alkaline conditions to cyanoacetic acid and ethanol (and so it does under most physiological and environmental conditions), while in acid pH the half life is considerably longer.
Knoevenagel condensation of ethyl cyanoacetate with aldehyde is reported. Microwave enhanced Knoevenegal condensation reaction of ethyl cyanoacetate with an aldehyde, P2O5, piperidine and chlorobenzene is reported.
Chemical Properties
Ethyl cyanoacetate is a colorless to straw colored liquid with a mild pleasant odor
Uses
Reagent used in labelled pyrimidine and purine synthesis. Ethyl cyanoacetate is an ester. It may be used in the synthesis of ethyl glyoxylate.It was used to investigate the Knoevenagel condensation reactions in microreactor using zeolite catalysts obtained by grafting amino groups onto NaX and CsNaX zeolites.
Preparation
Ethyl cyanoacetate can be prepared by the action of sodium or potassium cyanide on ethyl chloroacetate, and by the action of sodium cyanide on sodium chloroacetate, followed by esterification.
General Description
A colorless liquid. Denser than water. Contact may irritate skin, eyes and mucous membranes. Flash point 210°F. May be toxic by ingestion. Used to make other chemicals.
Air & Water Reactions
Slightly soluble in water.
Reactivity Profile
Ethyl cyanoacetate is both a nitrile and an ester. Esters react with acids to liberate heat along with alcohols and acids. Strong oxidizing acids may cause a vigorous reaction that is sufficiently exothermic to ignite the reaction products. Heat is also generated by the interaction of esters with caustic solutions. Flammable hydrogen is generated by mixing esters with alkali metals and hydrides. Nitriles may polymerize in the presence of metals and some metal compounds. They are incompatible with acids; mixing nitriles with strong oxidizing acids can lead to extremely violent reactions. Nitriles are generally incompatible with other oxidizing agents such as peroxides and epoxides. The combination of bases and nitriles can produce hydrogen cyanide. Nitriles are hydrolyzed in both aqueous acid and base to give carboxylic acids (or salts of carboxylic acids). These reactions generate heat. Peroxides convert nitriles to amides. Nitriles can react vigorously with reducing agents. Acetonitrile and propionitrile are soluble in water, but nitriles higher than propionitrile have low aqueous solubility. They are also insoluble in aqueous acids.
Hazard
Toxic by ingestion and inhalation.
Health Hazard
TOXIC; inhalation, ingestion or contact (skin, eyes) with vapors, dusts or substance may cause severe injury, burns or death. Contact with molten substance may cause severe burns to skin and eyes. Reaction with water or moist air will release toxic, corrosive or flammable gases. Reaction with water may generate much heat that will increase the concentration of fumes in the air. Fire will produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
Fire Hazard
Combustible material: may burn but does not ignite readily. Substance will react with water (some violently) releasing flammable, toxic or corrosive gases and runoff. When heated, vapors may form explosive mixtures with air: indoors, outdoors and sewers explosion hazards. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapors may travel to source of ignition and flash back. Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated or if contaminated with water.
Safety Profile
oison by ingestion. Moderately toxic by intraperitoneal and subcutaneous routes. Combustible when exposed to heat or flame; can react with oxidzing materials. Wdl react with water or steam to produce toxic and flammable vapors. To fight fire, use CO2, dry chemical. When heated to decomposition or on contact with acid or acid fumes it emits highly toxic fumes of CN-. See also NITRILES.
Potential Exposure
A nitrile used to manufacture dyes, pharmaceuticals, and other chemicals.
Shipping
UN3276 Nitriles, liquid, toxic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required, Potential Inhalation Hazard (Special Provision 5).
Purification Methods
Shake the ester several times with aqueous 10% Na2CO3, wash it well with water, dry with Na2SO4 and fractionally distil it. [Beilstein 2 IV 1889.]
Incompatibilities
Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides, and reducing agents. Nitriles may polymerize in the presence of metals and some metal compounds. They are incompatible with acids; mixing nitriles with strong oxidizing acids can lead to extremely violent reactions. Nitriles are generally incompatible with other oxidizing agents such as peroxides and epoxides. The combination of bases and nitriles can produce hydrogen cyanide. Nitriles are hydrolyzed in both aqueous acid and base to give carboxylic acids (or salts of carboxylic acids). These reactions generate heat. Peroxides convert nitriles to amides. Nitriles can react vigorously with reducing agents. Acetonitrile and propionitrile are soluble in water, but nitriles higher than propionitrile have low aqueous solubility. They are also insoluble in aqueous acids. Reacts with moisture, water, and steam, forming toxic fumes.
Waste Disposal
Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal.
Ethyl cyanoacetate Preparation Products And Raw materials
Raw materials
1of2
chevron_rightPreparation Products
1of8
chevron_right| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| ANJI BIOSCIENCES | +91-9000100077 +91-9000100077 | Hyderabad, India | 430 | 58 | Inquiry |
| Soham Chemical Industries | +91-7016081644 +91-7016081644 | Mumbai, India | 83 | 58 | Inquiry |
| JSK Chemicals | +919879767970 | Gujarat, India | 3756 | 58 | Inquiry |
| S D Fine Chem Limited | +91-9323715856 +91-9323715856 | Maharashtra, India | 368 | 58 | Inquiry |
| Associated Agencies | 91-22-28231752 | Maharashtra, India | 29 | 58 | Inquiry |
| Deepak Chemtex Private Limited | 08048372432Ext 456 | Mumbai, India | 20 | 58 | Inquiry |
| HiMedia Laboratories | 91-22-61471919 | Maharashtra, India | 1843 | 58 | Inquiry |
| Opulent Pharma | 08068441147Ext 981 | Gujarat, India | 810 | 58 | Inquiry |
| Advent Chembio Pvt., Ltd. | 91-22-27690836 | Maharashtra, India | 625 | 58 | Inquiry |
| Sahastraa Exports Pvt Ltd. | +91 22 66795555 | Mumbai, India | 2 | 58 | Inquiry |
| Supplier | Advantage |
|---|---|
| ANJI BIOSCIENCES | 58 |
| Soham Chemical Industries | 58 |
| JSK Chemicals | 58 |
| S D Fine Chem Limited | 58 |
| Associated Agencies | 58 |
| Deepak Chemtex Private Limited | 58 |
| HiMedia Laboratories | 58 |
| Opulent Pharma | 58 |
| Advent Chembio Pvt., Ltd. | 58 |
| Sahastraa Exports Pvt Ltd. | 58 |
105-56-6(Ethyl cyanoacetate)Related Search:
1of4
chevron_right




