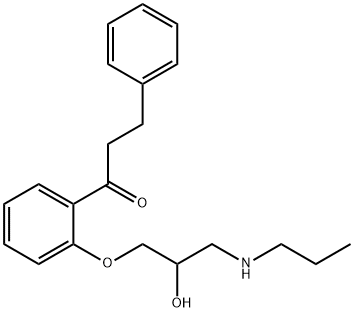
Propafenone
- CAS No.
- 54063-53-5
- Chemical Name:
- Propafenone
- Synonyms
- C07381;WZ-884-643;WZ-884-642;PROPAFENONE;AKOS 215-08;Propafenonum;Propafenone-d;Propafenone USP;(RS)-Propafenone;5-OH-Propafenone-D5
- CBNumber:
- CB2183169
- Molecular Formula:
- C21H27NO3
- Molecular Weight:
- 341.44
- MOL File:
- 54063-53-5.mol
- Modify Date:
- 2024/3/19 15:37:50
| Boiling point | 519.6±50.0 °C(Predicted) |
|---|---|
| Density | 1.096±0.06 g/cm3(Predicted) |
| storage temp. | 2-8°C |
| solubility | DMSO: 68 mg/mL (199.16 mM);Ethanol: 41 mg/mL (120.08 mM) |
| form | Solid |
| pka | pKa 9.27 (Uncertain) |
| color | White to Off-White |
| Water Solubility | 759.9ug/L(22.5 ºC) |
| CAS DataBase Reference | 54063-53-5(CAS DataBase Reference) |
| NIST Chemistry Reference | Propafenone(54063-53-5) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H302-H315-H319-H335 |
| Precautionary statements | P261-P280-P301+P312-P302+P352-P305+P351+P338 |
| Hazard Codes | T |
| Risk Statements | 46-22 |
| Safety Statements | 53-36/37/39-45 |
| WGK Germany | 3 |
| RTECS | UH2833000 |
Propafenone Chemical Properties,Uses,Production
Uses
Cardiac depressant (anti-arrhythmic).
Definition
ChEBI: An aromatic ketone that is 3-(propylamino)propane-1,2-diol in which the hydrogen of the primary hydroxy group is replaced by a 2-(3-phenylpropanoyl)phenyl group. It is a class 1C antiarrhythmic drug with local anesthetic effects, and is used as the hydroch oride salt in the management of supraventricular and ventricular arrhythmias.
World Health Organization (WHO)
Propafenone, a membrane-stabilizing antiarrhythmic agent, was introduced into medicine in the mid 1980s. Shortly afterwards, its use became associated with cases of severe cardiac arrhythmias, which led to notable restrictions in the drug's indications in at least two countries. See also WHO comment for flecainide.
General Description
Propafenone, 2-[2'-hydroxy-3-(propylamino)propoxy]-3-phenylpropiophenone (Rythmol), a classIC antiarrhythmic drug, contains a chiral center and is marketedas the racemic mixture. Therapy with the racemicmixture of propafenone produces effects that can be attributedto both (S) and (R) enantiomers. Although (R) and (S)enantiomers exert similarNa+channel–blocking effects, the(S) enantiomer also produces aβ-adrenergic blockade. As aresult, the (S) enantiomer is reported to be 40-fold morepotent than the (R) enantiomer as an antiarrhythmic agent.The enantiomers also display stereoselective dispositioncharacteristics. The (R) enantiomer is cleared more quickly.Hepatic metabolism is polymorphic and determined genetically.Ten percent of Caucasians have a reduced capacity tohydroxylate the drug to form 5-hydroxypropafenone. Thispolymorphic metabolism accounts for the interindividualvariability in the relationships between dose and concentrationand, thus, variability in the pharmacodynamic effects ofthe drug. The 5-hydroxy metabolites of both enantiomersare as potent as the parent compound in blocking Na+channels.Propafenone also depresses the slow inward current ofCa2+ions.
Clinical Use
Propafenone has been used for acute termination orlong-term suppression of ventricular arrhythmias. It isbound in excess of 95% to 1-acid glycoprotein in theplasma. It is absorbed effectively, but bioavailability is estimatedto be less than 20% because of first-pass metabolism.Less than 1% is eliminated as unchanged drug. Therapywith propafenone may produce effects that can be attributedto both (S) and (R) enantiomers. Thus, the effects may bemodulated because of an enantiomer–enantiomer interactionwhen patients are treated with the racemate.
Side effects
Concurrent administration of propafenone with
digoxin, warfarin, propranolol, or metoprolol increases
the serum concentrations of the latter four drugs.
Cimetidine slightly increases the propafenone serum
concentrations. Additive pharmacological effects can
occur when lidocaine, procainamide, and quinidine are
combined with propafenone.
As with other members of class IC, propafenone
may interact in an unfavorable way with other agents
that depress A-V nodal function, intraventricular conduction,
or myocardial contractility.
Overall, 21 to 32% of patients have adverse effects.
The most common are dizziness or light-headedness,
metallic taste, nausea, and vomiting; the most serious
are proarrhythmic events.
Precautions
Propafenone is contraindicated in the presence of severe or uncontrolled congestive heart failure; cardiogenic shock; sinoatrial, A-V, and intraventricular disorders of conduction; and sinus node dysfunction, such as sick sinus syndrome. Other contraindications include severe bradycardia, hypotension, obstructive pulmonary disease, and hepatic and renal failure. Because of its weak β-blocking action, propafenone may cause possible dose-related bronchospasm.This problem is greatest in patients who are slow metabolizers.
Propafenone Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| J S LABS | +91-7330612784 +91-7330612784 | Tamil Nadu, India | 160 | 58 | Inquiry |
| Humble Healthcare Limited | +91-9720093000 +91-8006400378 | Uttar Pradesh, India | 141 | 58 | Inquiry |
| Mylan Laboratories Ltd | +91-4023550543 +91-4030866666 | Telangana, India | 150 | 58 | Inquiry |
| Medilink Pharmachem | +91 (79) 3007-0133 | New Delhi, India | 424 | 50 | Inquiry |
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6351 | 58 | Inquiry |
| Pharmaffiliates Analytics and Synthetics P. Ltd | +91-172-5066494 | Haryana, India | 6773 | 58 | Inquiry |
| Sykem Pharma Resarch And Development | 08066085844Ext 735 | Maharashtra, India | 20 | 58 | Inquiry |
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 | China | 29798 | 60 | Inquiry |
| career henan chemical co | +86-0371-86658258 +8613203830695 | China | 29897 | 58 | Inquiry |
| Chongqing Chemdad Co., Ltd | +86-023-6139-8061 +86-86-13650506873 | China | 39916 | 58 | Inquiry |
54063-53-5(Propafenone)Related Search:
1of4
chevron_right




