Methotrexate
- CAS No.
- 59-05-2
- Chemical Name:
- Methotrexate
- Synonyms
- METHANOL-D4;MTX;Trexall;Methopterin;Abitrexate;sodium 4-[[4-[(2,4-diaminopteridin-6-yl)methyl-methyl-amino]benzoyl]am ino]-5-hydroxy-5-oxo-pentanoate;Rheumatrex;Methotrexat;Methoxtrexate;Methotextrate
- CBNumber:
- CB2739302
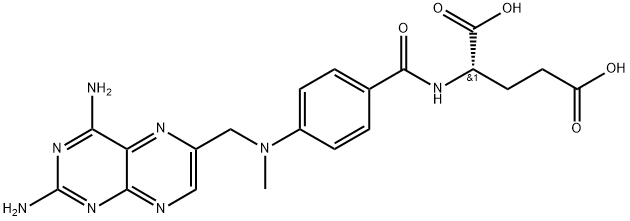
- Molecular Formula:
- C20H22N8O5
- Molecular Weight:
- 454.45
- MOL File:
- 59-05-2.mol
- MSDS File:
- SDS
- Modify Date:
- 2025/4/3 17:28:56
| Melting point | 195°C |
|---|---|
| Boiling point | 561.26°C (rough estimate) |
| alpha | +17~+24°(D/20℃)(c=1,Na2CO3 soln.)(calculated on the dehydrous basis) |
| Density | 1.4080 (rough estimate) |
| refractive index | 1.6910 (estimate) |
| Flash point | 11℃ |
| storage temp. | Keep in dark place,Inert atmosphere,Store in freezer, under -20°C |
| solubility | H2O: insoluble |
| form | powder |
| pka | pKa 3.04/4.99(H2O,t =25,I=0.0025) (Uncertain) |
| color | Light yellow to yellow |
| biological source | synthetic |
| Water Solubility | Insoluble. <0.1 g/100 mL at 19 ºC |
| Sensitive | Light Sensitive & Hygroscopic |
| Merck | 14,5985 |
| BRN | 70669 |
| BCS Class | 3 |
| Stability | Stable, but light sensitive and hygroscopic. Incompatible with strong acids, strong oxidizing agents. Store at -15C or below. |
| InChIKey | FBOZXECLQNJBKD-ZDUSSCGKSA-N |
| CAS DataBase Reference | 59-05-2(CAS DataBase Reference) |
| IARC | 3 (Vol. 26, Sup 7) 1987 |
| NIST Chemistry Reference | Methotrexate(59-05-2) |
| EPA Substance Registry System | Methotrexate (59-05-2) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS06,GHS08 |
|---|---|
| Signal word | Danger |
| Hazard statements | H301-H315-H319-H341-H360D |
| Precautionary statements | P201-P301+P310+P330-P302+P352-P305+P351+P338 |
| Hazard Codes | T,F |
| Risk Statements | 61-25-36/38-46-39/23/24/25-23/24/25-11 |
| Safety Statements | 53-26-36/37-45-36/37/39-36-16 |
| RIDADR | UN 2811 6.1/PG 3 |
| WGK Germany | 3 |
| RTECS | MA1225000 |
| F | 3-8-10 |
| TSCA | Yes |
| HazardClass | 6.1(b) |
| PackingGroup | III |
| HS Code | 29335995 |
| Hazardous Substances Data | 59-05-2(Hazardous Substances Data) |
| Toxicity | LD50 oral in rat: 135mg/kg |
Methotrexate price More Price(3)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | PHR1396 | Methotrexate Pharmaceutical Secondary Standard; Certified Reference Material | 59-05-2 | 1G | ₹9894.05 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | M-136 | Methotrexate solution 1.0?mg/mL in methanol with 0.1N NaOH, ampule of 1?mL, certified reference material, Cerilliant? | 59-05-2 | 1ML | ₹8738.1 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 454126 | Methotrexate - CAS 59-05-2 - Calbiochem | 59-05-2 | 100MG | ₹13960 | 2022-06-14 | Buy |
Methotrexate Chemical Properties,Uses,Production
Chemical Properties
Methotrexate is an orange-brown crystalline powder.
Uses
Methotrexate is used to treat severe lymphatic leukemia, choriocarcinoma, non-Hodgkin’s lymphoma, bone carcinoma, as well as head, neck, breast, and lung tumors.
Indications
Methotrexate is approved for use in severe disabling psoriasis recalcitrant to other less toxic treatments. The standard regimen is similar to low-dose therapy used for the treatment of rheumatoid arthritis . Although toxicities are similar to those described in the treatment of other diseases, hepatic cirrhosis and unexpected pancytopenia are of special concern given the chronicity of treatment.
Biological Functions
Although the mechanism of action of methotrexate in rheumatoid arthritis is unknown, recent studies have shown that methotrexate reversibly inhibits dihydrofolate reductase, blocking the proliferation of B cells by interfering with DNA synthesis, repair, and replication. Oral absorption is dose-dependent, being well-absorbed at doses of 7.5–25 mg once a week. At this dose, oral bioavailability is approximately 60%, and food can delay absorption and reduce peak concentration. The volume of distribution is 0.4 to 0.8 L/kg. Protein binding is approximately 50%. It is metabolized to active metabolites, methotrexate polyglutamates and 7-hydroxymethotrexate. Some metabolism occurs by intestinal flora after oral administration. Methotrexate is actively transported into the urine (80–90% unchanged in the urine within 24 hours) via the folate transporter, an organic anion transporter. Its elimination half-life is 3 to 10 hours.
Acquired resistance
Mammalian cells have several mechanisms of resistance
to methotrexate. These include an increase in intracellular
dihydrofolate reductase levels, appearance
of altered forms of dihydrofolate reductase with decreased
affinity for methotrexate, and a decrease in
methotrexate transport into cells. The
relative importance of each of these mechanisms of resistance
in various human tumors is not known.
Cellular uptake of the drug is by carrier-mediated
active transport. Drug resistance due to decreased
transport can be overcome by greatly increasing extracellular
methotrexate concentration, which provides a
rationale for high-dose methotrexate therapy. Since
bone marrow and gastrointestinal cells do not have impaired
folate methotrexate transport, these normal cells
can be selectively rescued with reduced folate, bypassing
the block of dihydrofolate reductase. Leucovorin
(citrovorum factor, folinic acid, 5-formyltetrahydrofolate)
is the agent commonly used for rescue.
General Description
Methotrexate (MTX, Rheumatrex), an antifolate drug used in cancer treatment, has also been used in the disease management of RA since the 1950s. Because of its quicker therapeutic onset among all DMARDs and its demonstrated efficacy, tolerability, and low cost, MTX has been the firstline therapy for RA patients who are not responsive to NSAIDs alone.
Recent findings have indicated that other DMARDs should only be used for patients who are refractory to MTX. At least four anti-inflammatory mechanisms of action have been suggested for MTX’s ability to slow down RA disease progression. First, MTX, being a folate antagonist, prevents antigen-dependent T-cell proliferation by blocking de novo pyrimidine biosynthesis, via a reversible inhibition of dihydrofolate reductase. It also inhibits folate-mediated production of spermine and spermidine in synovial tissue. These polyamines are believed to be the toxic compounds responsible for causing tissue injury in RA. MTX can also reduce intracellular glutathione concentration, thereby altering the cellular redox state that suppresses the formation of reactive oxygen radicals in synovial tissue. Lastly, MTX, similar to sulfasalazine, infliximab, and IL-4, can also inhibit osteoclastogenesis (i.e., bone erosion) in patients with RA, by modulating the interaction of the receptor activator of nuclear factor B, its ligand, and osteoprotegrin.
Air & Water Reactions
Methotrexate is sensitive to hydrolysis, oxidation and light. Insoluble in water.
Reactivity Profile
Methotrexate decomposes in very acidic or alkaline conditions. Methotrexate is incompatible with strong oxidizing agents and strong acids.
Hazard
Very toxic. Questionable carcinogen.
Fire Hazard
Flash point data for Methotrexate are not available; however, Methotrexate is probably combustible.
Biological Activity
Cytotoxic agent. Inhibits thymidylate synthetase and de novo purine synthesis. Potent folic acid antagonist; inhibits dihydrofolate reductase. Also inhibits Ras carboxyl methylation in DKOB8 cells, leading to decreased p44 and Akt activation.
Mechanism of action
Methotrexate is a folic acid antagonist structurally designed to compete successfully with 7,8-DHF for the DHFR enzyme. The direct inhibition of DHFR causes cellular levels of 7,8-DHF to build up, which in turn results in feedback (indirect) inhibition of thymidylate synthase. Methotrexate also is effective in inhibiting glycine amide ribonucleotide (GAR) transformylase , a key enzyme in the synthesis of purine nucleotides. Take note of the structural differences between methotrexate and DHF, because these differences will be important to an understanding of the chemical mechanism of this anticancer agent.
Pharmacology
Methotrexate is a folate antimetabolite that inhibits dihydrofolate reductase and other folate-dependent enzymes in cells. At the low doses used in the therapy of rheumatoid arthritis,methotrexate appears to be acting more as an antiinflammatory agent than as an immunosuppressant. Methotrexate inhibits folate-dependent enzymes involved in adenosine degradation, increasing concentrations of extracellular adenosine. Adenosine acts via cell surface receptors to inhibit the production of inflammatory cytokines such as TNF-α and IFN-γ.Methotrexate also decreases the production of inflammatory prostaglandins and proteases, though a direct action on the COX enzymes has not been noted.
Clinical Use
Methotrexate is part of curative combination
chemotherapy for acute lymphoblastic leukemias,
Burkitt’s lymphoma, and trophoblastic choriocarcinoma.
It is also useful in adjuvant therapy of breast carcinoma;
in the palliation of metastatic breast, head, neck,
cervical, and lung carcinomas; and in mycosis fungoides.
High-dose methotrexate administration with leucovorin
rescue has produced remissions in 30% of patients
with metastatic osteogenic sarcoma.
Methotrexate is one of the few anticancer drugs that
can be safely administered intrathecally for the treatment
of meningeal metastases. Its routine use as prophylactic
intrathecal chemotherapy in acute lymphoblastic
leukemia has greatly reduced the incidence
of recurrences in the CNS and has contributed to the
cure rate in this disease. Daily oral doses of methotrexate
are used for severe cases of the nonneoplastic skin
disease psoriasis, and methotrexate
has been used as an immunosuppressive agent in severe
rheumatoid arthritis.
Side effects
In the low-dose regimen used for rheumatoid arthritis, most side effects of methotrexate are mild and can be managed by temporarily stopping the drug or reducing the dose. These include nausea, stomatitis, GI discomfort, rash, diarrhea, and headaches. Changes in liver aminotransferases and mild to moderate immunosuppression have been reported in rheumatoid arthritis patients taking methotrexate. Severe toxicity is possible but rare and may be a function of drug accumulation. These effects include hepatotoxicity progressing to cirrhosis, pneumonitis progressing to pulmonary fibrosis, and bone marrow depression with anemia, leukopenia, and thrombocytopenia. Folic acid supplementation is often used to alleviate certain side effects of methotrexate therapy (stomatitis, GI irritation, hematopoietic effects) but may also contribute to resistance to this therapy.
Potential Exposure
Methotrexate is an alkaloid anticancer drug available in tablet or injectable liquid form. A chemotherapy drug that interferes with DNA and RNA synthesis. It is also an insect chemosterilant.
Metabolism
Methotrexate can be given orally in the treatment of breast, head and neck, and various lung cancers as well as in non-Hodgkin's lymphoma. The sodium salt form also is marketed for IV, intramuscular, intra-arterial, or intrathecal injection. Oral absorption is dose-dependent and peaks at 80 mg/m2 because of site saturation. The monoglutamate tail of methotrexate permits active transport into cells, with carrier-mediated transport predominating at serum concentration levels lower than 100 μM. Once inside the cell, methotrexate undergoes a polyglutamation reaction that adds several anionic carboxylate groups to trap the drug at the site of action. Polyglutamation is more efficient in tumor cells than in healthy cells and, therefore, may promote selective toxicity of this drug. Cancer cells can become resistant to methotrexate over time which may involve impaired transport across tumor cell membranes, enhanced efflux from the tumor cell, and attenuated polyglutamation rates. The polyglutamated drug will be hydrolyzed back to the parent structure before renal elimination. Up to 90% of an administered dose of methotrexate is excreted unchanged in the urine within 24 hours.
Shipping
UN1544 Alkaloids, solid, n.o.s. or Alkaloid salts, solid, n.o.s. poisonous, Hazard Class: 6.1; Labels: 6.1- Poisonous materials, Technical Name Required. UN2811 Toxic solids, organic, n.o.s., Hazard Class: 6.1; Labels: 6.1- Poisonous materials, Technical Name Required.
Purification Methods
Most common impurities are 10-methylpteroylglutamic acid, aminopterin and pteroylglutamic acid. Purify it by chromatography on Dowex-1 acetate, followed by filtration through a mixture of cellulose and charcoal. It has been recrystallised from aqueous HCl or by dissolution in the minimum volume of N NaOH and acidified until precipitation is complete, filter or better collect by centrifugation, wash with H2O (also by centrifugation) and dry at 100o/3mm. It has UV: max at 244 and 307nm ( 17300 and 19700) in H2O at pH 1; 257, 302 and 370nm ( 23000, 22000 and 7100) in 2O at pH 13. [Momle Biochemical Preparations 8 20 1961, Seeger et al. J Am Chem Soc 71 1753 1949.] It is a potent inhibitor of dihydrofolate reductase and is used in cancer chemotherapy. [Blakley The Biochemistry of Folic Acid and Related Pteridines, North-Holland Publ Co., Amsterdam, NY, pp157-163 1969, Beilstein 26 IV 3833.] It is CARCINOGENIC; HANDLE WITH EXTREME CARE.
Incompatibilities
Combustible. Compounds of the carboxyl group react with all bases, both inorganic and organic (i.e., amines) releasing substantial heat, water and a salt that may be harmful. Incompatible with arsenic compounds (releases hydrogen cyanide gas), diazo compounds, dithiocarbamates, isocyanates, mercaptans, nitrides, and sulfides (releasing heat, toxic, and possibly flammable gases), thiosulfates and dithionites (releasing hydrogen sulfate and oxides of sulfur). Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides, light, UV, moisture.
Waste Disposal
It is inappropriate and possibly dangerous to the environment to dispose of expired or waste drugs and pharmaceuticals by flushing them down the toilet or discarding them to the trash. Household quantities of expired or waste pharmaceuticals may be mixed with wet cat litter or coffee grounds, double-bagged in plastic, discard in trash. Larger quantities shall carefully take into consideration applicable DEA, EPA, and FDA regulations. If possible return the pharmaceutical to the manufacturer for proper disposal being careful to properly label and securely package the material. Alternatively, the waste pharmaceutical shall be labeled, securely packaged and transported by a state licensed medical waste contractor to dispose by burial in a licensed hazardous or toxic waste landfill or incinerator.
Precautions
Methotrexate is teratogenic and is contraindicated duringpregnancy and breast-feeding. Prior to attemptingpregnancy, women should wait at least one menstrualcycle and men at least 3 months after discontinuing thisdrug. Additional contraindications to methotrexate administrationinclude kidney, liver, and lung disease;moderate to high alcohol use; immunodeficiency; blooddyscrasias; and hypersensitivity. Elderly persons may be at increased risk for toxicity because of decreased renaland hepatic function.
Methotrexate clearance can be decreased by thecoadministration of NSAIDs; however, this not usuallya problem with the low doses of methotrexate used totreat arthritis. Methotrexate can be displaced fromplasma protein binding sites by phenylbutazone, phenytoin,sulfonylureas, and sulfonamides and certain otherantibiotics. The antifolate effects of methotrexate areadditive with those of other folate-inhibitory drugs,such as trimethoprim.
Methotrexate Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Jigs Chemical ltd | +919099003427 | Gujarat, India | 239 | 58 | Inquiry |
| Bulat Pharmaceutical Pvt Ltd | +91-8448085659 +91-8448085660 | Haryana, India | 123 | 58 | Inquiry |
| Frolic Pharmachem | +919711094561 | Himachal Pradesh, India | 94 | 58 | Inquiry |
| Avra Laboratories Pvt Ltd | +91-8008572391 +91-8008572391 | Hyderabad, India | 43 | 58 | Inquiry |
| Vannsh Life Sciences Pvt Ltd | +91-4023555246 +91-9642555246 | Hyderabad, India | 15 | 58 | Inquiry |
| Svak Life Sciences | +91-9963463333 +91-9705383777 | Hyderabad, India | 101 | 58 | Inquiry |
| Global Calcium Pvt Ltd | +91-8050750904 +91-8040554500 | Karnataka, India | 132 | 58 | Inquiry |
| ALS INDIA LIFE SCIENCES | +91-8977036379 +91-8977036379 | Hyderabad, India | 1878 | 58 | Inquiry |
| Ralington Pharma | +91-7948911722 +91-9687771722 | Gujarat, India | 1350 | 58 | Inquiry |
| Beta Drugs | +91-9316590666 +91-7015991921 | Haryana, India | 34 | 58 | Inquiry |
| Supplier | Advantage |
|---|---|
| Jigs Chemical ltd | 58 |
| Bulat Pharmaceutical Pvt Ltd | 58 |
| Frolic Pharmachem | 58 |
| Avra Laboratories Pvt Ltd | 58 |
| Vannsh Life Sciences Pvt Ltd | 58 |
| Svak Life Sciences | 58 |
| Global Calcium Pvt Ltd | 58 |
| ALS INDIA LIFE SCIENCES | 58 |
| Ralington Pharma | 58 |
| Beta Drugs | 58 |
Related articles
- Methotrexate: Mechanism of Action and Mechanisms of Resistance
- Methotrexate can inhibit dihydrofolate reductase (DHFR, Ki = 10?11 M), a key enzyme for the maintenance of biologically active....
- Mar 16,2024
59-05-2(Methotrexate)Related Search:
1of4
chevron_right




