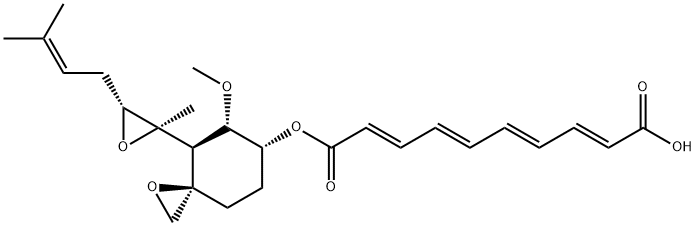
Fumagillin
- CAS No.
- 23110-15-8
- Chemical Name:
- Fumagillin
- Synonyms
- h-3;fugilin;fumidil;CS-1349;NSC 9168;FUGILLIN;FUMADIL B;FUMIDIL B;FUMAGILLIN;AMEBACILIN
- CBNumber:
- CB3142925
- Molecular Formula:
- C26H34O7
- Molecular Weight:
- 458.54
- MOL File:
- 23110-15-8.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/6/21 13:10:12
| Melting point | 194-195℃ |
|---|---|
| alpha | D25 -26.6° (c = 1 in 95% ethanol) |
| Boiling point | 484.03°C (rough estimate) |
| Density | 1.1368 (rough estimate) |
| refractive index | 1.5800 (estimate) |
| Flash point | 2℃ |
| storage temp. | 2-8°C |
| solubility | ethanol: 1 mg/mL |
| form | powder |
| pka | 4.27±0.10(Predicted) |
| color | white |
| Sensitive | Air Sensitive |
| Stability | Stable for 1 year from date of purchase as supplied. Solutions in DMSO may be stored at -20°C for up to 3 months. |
| InChIKey | NGGMYCMLYOUNGM-ZILXAVPASA-N |
| LogP | 3.260 (est) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H302 | |||||||||
| Precautionary statements | P264-P270-P301+P312-P501 | |||||||||
| Hazard Codes | Xn,F | |||||||||
| Risk Statements | 22-36-20/21/22-11 | |||||||||
| Safety Statements | 36-36/37-16-24/25 | |||||||||
| RIDADR | UN 1648 3 / PGII | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | HE1750000 | |||||||||
| HS Code | 29419090 | |||||||||
| Toxicity | LD50 in mice (mg/kg): ~800 s.c. (DiPaolo) | |||||||||
| NFPA 704 |
|
Fumagillin price More Price(4)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | F6771 | Fumagillin from Aspergillus fumigatus ≥90%, powder | 23110-15-8 | 1MG | ₹27950.15 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | F6771 | Fumagillin from Aspergillus fumigatus ≥90%, powder | 23110-15-8 | 5MG | ₹100910.65 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 37017 | Fumagillin solution ~100?μg/mL in acetonitrile, analytical standard | 23110-15-8 | 1ML | ₹54410.3 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 344845 | Fumagillin, Aspergillus fumigatus - CAS 23110-15-8 - Calbiochem Antiprotozoal agent and angiogenesis inhibitor. | 23110-15-8 | 500μG | ₹13100 | 2022-06-14 | Buy |
Fumagillin Chemical Properties,Uses,Production
Description
Fumagillin is a fungal metabolite that has been found in A. fumigatus and has diverse biological activities. It inhibits methionyl aminopeptidase 2 (METAP2; IC50 = 10 nM). Fumagillin (10 ng/ml) inhibits tube formation in a rat blood vessel organ culture assay. It inhibits E. cuniculi replication in isolated rabbit kidney cells and canine embryo cells when used at a concentration of 5 μg/ml. In vivo, fumagillin (30 mg/kg per day) decreases tumor growth and the number of metastases in a mouse model of diethylnitrosamine-induced hepatocellular carcinoma. It also reduces subcutaneous and gonadal fat mass in a mouse model of high-fat diet-induced obesity. Formulations containing fumagillin have been used to treat conjunctival and intestinal microsporidial infections in immunocompromised patients.
Chemical Properties
White powder
Uses
Fumagillin is a compound isolated from the fungus Aspergillus fumigatus. Fumagillin is an antimicrobial agent used in the treatment of microsporidiosis. Fumagillin shows promise as both an an anti-inf ective and antiangiogenic agent.
Definition
An antibiotic substance produced by Aspergillus fumigatus.
General Description
Fumagillin is an antibiotic obtained by the fermentation of certain strains of Aspergillus fumigatus with potent amoebicidal action.
Biological Activity
Antibiotic and antiangiogenic agent; covalently binds and inhibits methionine aminopeptidase-2. Inhibits endothelial cell proliferation in vitro and tumor-induced angiogenesis in vivo . Also inhibits tumor growth in mice. Analog available, TNP 470 (N-(2-Chloroacetyl)carbamic acid (3R,4S,5S,6R)-5-Methoxy-4-[(2R,3R)-2-methyl-3-(3-methyl -2-buten-1-yl)-2-oxiranyl]-1-oxaspiro[2.5]oct-6-yl ester).
Purification Methods
Forty grams of a commercial sample containing 42% fumagillin, 45% sucrose, 10% antifoam agent and 3% of other impurities are digested with 150mL of CHCl3. The insoluble sucrose is filtered off and washed with CHCl3. The combined CHCl3 extracts are evaporated almost to dryness at room temperature under reduced pressure. The residue is triturated with 20mL of MeOH, and the fumagillin is filtered off by suction. It is crystallised twice from 500mL of hot MeOH by standing overnight in a refrigerator (yellow needles). (The long-chain fatty ester used as antifoam agent is still present, but is then removed by repeated digestion, on a steam bath, with 100mL of diethyl ether.) For further purification, the fumagillin (10g) is dissolved in 150mL of 0.2M ammonia, and the insoluble residue is filtered off. The ammonia solution (cooled in running cold water) is then brought to pH 4 by careful addition of M HCl with constant shaking in the presence of 150mL of CHCl3. (Fumagillin is acid-labile and must be removed rapidly from the aqueous acid solution.) The CHCl3 extract is washed several times with distilled water, dried (Na2SO4) and evaporated under reduced pressure. The solid residue is washed with 20mL of MeOH. The fumagillin is filtered off by suction, then crystallised from 200mL of hot MeOH. [Tarbell et al. J Am Chem Soc 77 5610 1955.] Alternatively, 10g of fumagillin in 100mL CHCl3 is passed through a silica gel (5g) column to remove tarry material, and the CHCl3 is evaporated to leave an oil which gives fumagillin on crystallisation from amyl acetate. It recrystallises from MeOH (charcoal) or Me2CO/MeOH. The fumagillin is stored in dark bottles in the absence of oxygen and at low temperatures. [Schenk et al. J Am Chem Soc 77 5606 1955, Beilstein 19 III/IV 1012.]
Fumagillin Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Pharmaffiliates Analytics and Synthetics P. Ltd | +91-172-5066494 | Haryana, India | 6773 | 58 | Inquiry |
| Pharma Affiliates | 172-5066494 | Haryana, India | 6761 | 58 | Inquiry |
| Clearsynth Labs | 91-22-45045900 | Maharashtra, India | 3889 | 58 | Inquiry |
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6351 | 58 | Inquiry |
| Shaanxi Cuikang Pharmaceutical Technology Co., Ltd | +86-19164747840 +86-13119157289 | China | 2969 | 58 | Inquiry |
| Hebei Mojin Biotechnology Co., Ltd | +86 13288715578 +8613288715578 | China | 12460 | 58 | Inquiry |
| Ouhuang Engineering Materials (Hubei) Co., Ltd | +86-86177 | China | 2952 | 58 | Inquiry |
| Apeloa production Co.,Limited | +8619933239880 | China | 853 | 58 | Inquiry |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | China | 21675 | 55 | Inquiry |
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 | China | 32760 | 60 | Inquiry |
Related articles
- What are the effects of fumagillin on bees?
- Fumagillin is the only antibiotic approved for controlling nosema disease in honey bees. It has been extensively used in Unite....
- Mar 25,2024
23110-15-8(Fumagillin)Related Search:
1of4
chevron_right




