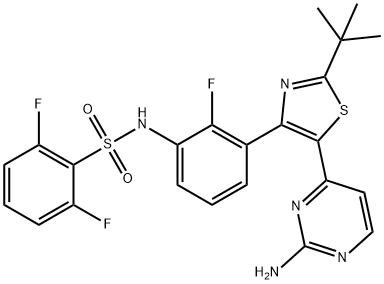
Dabrafenib
- CAS No.
- 1195765-45-7
- Chemical Name:
- Dabrafenib
- Synonyms
- Dabrafenib (GSK2118436);N-[3-[5-(2-Amino-4-pyrimidinyl)-2-(tert-butyl)-4-thiazolyl]-2-fluorophenyl]-2,6-difluorobenzenesulfonamide;N-[3-[5-(2-aminopyrimidin-4-yl)-2-tert-butyl-1,3-thiazol-4-yl]-2-fluorophenyl]-2,6-difluorobenzenesulfonamide;CS-658;abrafenib;Dabrafenib;Darla fini;GSK2118436A;Debrafenib API;Dabrafenib Base
- CBNumber:
- CB32604230
- Molecular Formula:
- C23H20F3N5O2S2
- Molecular Weight:
- 519.56
- MOL File:
- 1195765-45-7.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/5/25 11:23:34
| Melting point | 214-216oC |
|---|---|
| Boiling point | 653.7±65.0 °C(Predicted) |
| Density | 1.443 |
| storage temp. | -20°C |
| solubility | Soluble in DMSO (up to 30 mg/ml with warming), or in Ethanol (up to 1 mg/ml with warming). |
| form | White solid. |
| pka | 6.62±0.10(Predicted) |
| color | Off-white |
| Stability | Stable for 1 year from date of purchase as supplied. Solutions in DMSO or ethanol may be stored at -20°C for up to 3 months. |
| InChIKey | BFSMGDJOXZAERB-UHFFFAOYSA-N |
| SMILES | C1(S(NC2=CC=CC(C3=C(C4C=CN=C(N)N=4)SC(C(C)(C)C)=N3)=C2F)(=O)=O)=C(F)C=CC=C1F |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS08,GHS09 |
|---|---|
| Signal word | Warning |
| Hazard statements | H361-H411-H400 |
| Precautionary statements | P273-P391-P501-P201-P202-P281-P308+P313-P405-P501 |
| HS Code | 29350090 |
Dabrafenib Chemical Properties,Uses,Production
Description
In May 2013, the US FDA approved dabrafenib (also referred to as GSK 2118436) for the treatment of patients with unresectable or metastatic melanoma with the BRAFV600E mutation as detected by a FDA-approved test. Dabrafenib was identified from a screen of an oncology-directed kinase collection, followed by extensive structure–activity relationships (SAR) on an initial thiazole lead. Dabrafenib is a potent inhibitor of B-BRAFV600E kinase (IC50=0.65 nM) compared to its potency against wild-type B-raf (IC50=3.2 nM). It also inhibits other kinases (e.g., CRAF) and other mutant B-raf kinases (BRAFV600E and BRAFV600D) with enzyme IC50s of <5 nM and is fairly selective versus a panel of 270 kinases. Consistent with its in vitro activity, oral administration of dabrafenib inhibits the growth of B-RafV600E mutant melanoma (A375P) and colon cancer (Colo205) human tumor xenografts growing subcutaneously in immunocompromised mice. Key steps in the synthesis of dabrafenib are condensation of an aryl sulfonamide ester with the lithium anion of 2-chloro-4-methylpyrimidine to generate a ketone intermediate and bromination of the ketone intermediate with N-bromosuccinamide followed by cyclization with tert-butyl thioamide to afford the desired thiazole core.
Uses
Dabrafenib is an inhibitor of mutated BRAF kinase and has clinical activity with a manageable safety profile in clinical trials of phase 1 and 2 in patients with BRAF(V600)-mutated metastatic melanoma.
Definition
ChEBI: An organofluorine compound and antineoplastic agent, used as its mesylate salt in treatment of metastatic melanoma.
General Description
Class: dual threonine/tyrosine kinase; Treatment: melanoma with BRAF mutations; Oral bioavailability = 95%; Elimination half-life = 8 h; Protein binding = 99.7%
Pharmacokinetics
Dabrafenib exhibits an oral bioavailability of
95%, indicative of extensive absorption and low firstpass intestinal and hepatic metabolism. The
excellent oral bioavailability contributes to a much
lower dosage than vemurafenib (150 mg, BID vs.
960 mg, BID). It has an elimination half-life of 8 h,
resulting in twice-daily dosing regimen. Dabrafenib undergoes metabolism primarily via
oxidation of the t-butyl group to form hydroxydabrafenib 6, which is further oxidized to carboxydabrafenib 7. Subsequent decarboxylation furnishes
the desmethyl-dabrafenib 8 via a pH-dependent
decarboxylation (Fig. 4). The major route of
elimination of dabrafenib is a combination of
oxidative metabolism (48% of the dose) and biliary
excretion.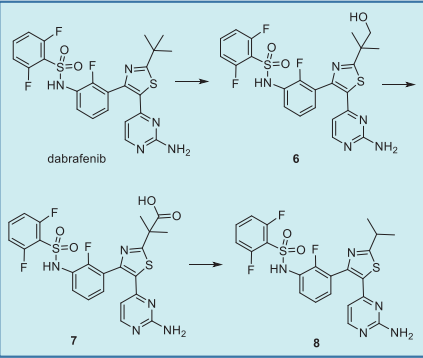
Dabrafenib Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| AVD pharmaceuticals Pvt Ltd | +919860835260 | Pune, India | 102 | 58 | Inquiry |
| Venkatasai Life Sciences | +91-9908134868 +91-8008303069 | Hyderabad, India | 186 | 58 | Inquiry |
| Aspen Biopharma Labs Pvt Ltd | +91-9248058660 +91-9248058662 | Telangana, India | 234 | 58 | Inquiry |
| AKASH PHARMA EXPORTS | +91-9388123451 +91-9846039283 | Kerela, India | 470 | 58 | Inquiry |
| Pharma Affiliates | 172-5066494 | Haryana, India | 6761 | 58 | Inquiry |
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6351 | 58 | Inquiry |
| A.J Chemicals | 91-9810153283 | New Delhi, India | 6124 | 58 | Inquiry |
| Pharmaffiliates Analytics and Synthetics P. Ltd | +91-172-5066494 | Haryana, India | 6773 | 58 | Inquiry |
| Zison Pharmaceutical (Shandong) Co., Ltd. | +86-0086-531-88259693 +86-18660188356 | China | 57 | 58 | Inquiry |
| Henan Bao Enluo International TradeCo.,LTD | +86-17331933971 +86-17331933971 | China | 2503 | 58 | Inquiry |





