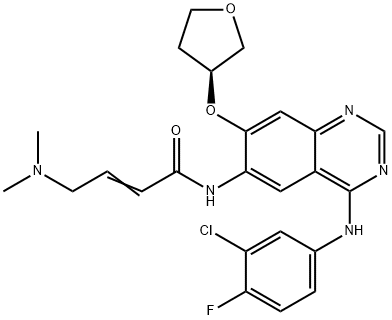
Afatinib (BIBW 2992)
- CAS No.
- 439081-18-2
- Chemical Name:
- Afatinib (BIBW 2992)
- Synonyms
- Afatinib Base;BIBW 2992;(E)-N-[4-(3-chloro-4-fluoroanilino)-7-[(3S)-oxolan-3-yl]oxyquinazolin-6-yl]-4-(dimethylamino)but-2-enamide;N-[4-[(3-Chloro-4-fluorophenyl)amino]-7-[[(3S)-tetrahydro-3-furanyl]oxy]-6-quinazolinyl]-4-(dimethylamino)-2-butenamide;2992;Tovok;CS-95;Afatinb;ToMtovok;BIBW-2292
- CBNumber:
- CB12470923
- Molecular Formula:
- C24H25ClFN5O3
- Molecular Weight:
- 485.94
- MOL File:
- 439081-18-2.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/7/2 8:55:00
| Boiling point | 676.9±55.0 °C(Predicted) |
|---|---|
| Density | 1.380 |
| storage temp. | Store at -20°C |
| solubility | ≥24.3 mg/mL in DMSO; insoluble in H2O; ≥42.1 mg/mL in EtOH with ultrasonic |
| form | solid |
| pka | 12.06±0.43(Predicted) |
| color | White to off-white |
| CAS DataBase Reference | 439081-18-2 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H302-H315-H319-H335 |
| Precautionary statements | P261-P305+P351+P338 |
| HS Code | 29349990 |
Afatinib (BIBW 2992) Chemical Properties,Uses,Production
Description
In July 2013, the US FDA approved afatinib (also referred to as BIBW- 2992), for the first-line treatment of patients with metastatic non-small cell lung cancer (NSCLC) and with tumors that have epidermal growth factor receptor (EGFR) exon 19 deletions or exon 21 (L858R) substitution. Afatinib functions as an irreversible inhibitor by covalently binding directly to the ATP-binding site in the kinase domains of both EGFR(Cys 773) andHER2 (Cys 805;HER-2 is the preferred dimerization partner of EGFR) resulting in downregulation of EGFR signaling. Afatinib is a potent inhibitor of wild-type and mutant forms (L858R) of EGFR (IC50s of 0.5 and 0.4 nM, respectively), and HER2 (IC50=14 nM), but about 100-fold more active against the gefitinib resistant L858R– T790M EGFR double mutant, with an IC50 of 10 nM. Consistent with its in vitro activity, afatinib induces tumor regression in xenograft and transgenic lung cancer models, with superior activity over erlotinib. A synthetic route to afatinib that employs the displacement of a phenylsulfonyl group to install the (S)-3-hydoxytetrahydrofuran ring and a modified Horner–Wadsworth–Emmons reaction with {[4-(3-chloro-4- phenylamino)-7-((S)-tetrahydrofuran-3-yloxy)-quinazolin-6-ylcarbamoyl]- methyl}-phosphonate and dimethylaminoacetaldehyde-hydrogen sulfite adduct to install the eneamide moiety, has been reported.
Uses
BIBW2992 (Afatinib, Tomtovok, Tovok) irreversibly inhibits EGFR/HER2 including EGFRwt, EGFRL858R, EGFRL858R/T790M and HER2 with IC50 of 0.5 nM, 0.4 nM, 10 nM and 14 nM, respectively.
Afatinib (BIBW 2992) Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| DHANVI EXIM SERVICES | +91-8790148237 +91-8790148237 | Hyderabad, India | 102 | 58 | Inquiry |
| A.J Chemicals | 91-9810153283 | New Delhi, India | 6124 | 58 | Inquiry |
| Hebei Mojin Biotechnology Co., Ltd | +86 13288715578 +8613288715578 | China | 12447 | 58 | Inquiry |
| Hebei Yanxi Chemical Co., Ltd. | +86-17531190177; +8617531190177 | China | 5873 | 58 | Inquiry |
| shandong perfect biotechnology co.ltd | +86-53169958659; +8618596095638 | China | 294 | 58 | Inquiry |
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 | China | 20294 | 58 | Inquiry |
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 | China | 29798 | 60 | Inquiry |
| Shanghai Bojing Chemical Co.,Ltd. | +86-86-02137122233 +8613795318958 | China | 299 | 55 | Inquiry |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | China | 21667 | 55 | Inquiry |
| Hangzhou FandaChem Co.,Ltd. | 008657128800458; +8615858145714 | China | 9338 | 55 | Inquiry |
439081-18-2(Afatinib (BIBW 2992))Related Search:
1of4
chevron_right




